Background
Strongyloidiasis is an intestinal infection caused by two species of the parasitic nematode Strongyloides. The most common and clinically important pathogenic species in humans is S stercoralis (see the following image). S fuelleborni is found sporadically in Africa and Papua New Guinea. Distinctive characteristics of this parasite are its ability to persist and replicate within a host for decades while producing minimal or no symptoms in immune competent individuals and its potential to cause life-threatening infection (hyperinfection syndrome, disseminated strongyloidiasis) in an immunocompromised host (60%-85% mortality rate). [1, 2, 3, 4]
The symptoms related to strongyloidiasis may reflect the nematode's systemic passage, its local cutaneous involvement, or both. Infection is clinically characterized by watery diarrhea, abdominal cramping, and urticarial rash. In malnourished children, strongyloidiasis remains an important cause of chronic diarrhea, cachexia, and failure to thrive. During chronic uncomplicated infections, the larvae may migrate to the skin, where they can cause cutaneous strongyloidiasis, known as larva currens because of the quick migratory rate of the larva.
This condition can also be a health consequence of captivity. During World War II, Allied military personnel held by the Japanese experienced deprivation, malnutrition, and exposure to tropical diseases. [5] Certain tropical diseases have persisted in these survivors, notably infections with S stercoralis, with studies conducted 30 years or more after release from captivity documenting overall infection rates of 15%. Chronic strongyloidiasis may produce a linear urticarial larva currens rash, with such individuals at risk for fatal hyperinfection if immunity is suppressed. [1]
Patient Education
Travelers to endemic areas should wear footwear when walking on the beach and other areas with soil. Community education in endemic areas should include sewage management; avoidance of soil contaminated with feces or the use of feces for fertilizer; wearing of protective clothing when handling sewage or contaminated soil; and wearing of shoes while outdoors.
Pathophysiology
The life cycle of Strongyloides stercoralis is complex and unique among the intestinal nematodes. This worm has two types of life cycles—a free-living life cycle (rhabditiform larvae) and a parasitic life cycle (filariform infective larvae)—with three developmental stages: adult, rhabditiform larva, and filariform larva.
The first type of life cycle allows development of nonparasitic adults, both males and females, in the soil, which can indefinitely maintain infestation of the soil. This free-living phase is occasionally termed the heterogonic life cycle.
The second type of life cycle allows noninfective new larvae to molt in the human host into infective filariform larvae. Infective larvae can penetrate the intestine and set up a new cycle, commonly termed the hyperinfective or autoinfective cycle. In this setting, unlike in other intestinal nematodes of humans, the larvae can increase in numbers without reinfection from outside. This life-cycle variation is responsible for the decades-long persistence of infection in untreated hosts.
The adult female worm is a minute, slender, almost transparent worm that measures approximately 2.2 mm to 2.5 mm long and has a diameter of 50 µm. The adult female worm lives in tunnels between the enterocytes in the small bowel of humans.
A parasitic male exists, but it is found only in experimentally infected dogs and has no role in human infections. Parasitic males are shorter and broader than females and are easily eliminated from the intestine. Only adult females are found in infected humans.
Humans are the principal host of S stercoralis. Dogs, cats, and other mammals can also harbor the worm and may serve as reservoir hosts.
Stage 1
Human infection is acquired by penetration of the skin or mucous membranes by infective filariform larvae, either from autoinfection or from contact with infected soil or other material contaminated with human feces (fecal-oral route) (see the image below). This is facilitated by a potent histolytic protease that is secreted by the organism. At the portal of entry, the larvae cause petechial hemorrhages, which are accompanied by intense pruritus, congestion, and edema.
Stage 2
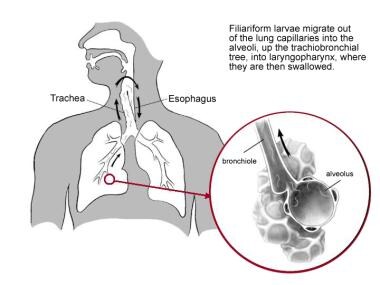
Migration of infective larvae traditionally has been believed to occur via the lymphatic vessels and venules. Eventually the larvae reach the pulmonary circulation, where, in the pulmonary capillaries, the larvae produce hemorrhages, which form the avenue of penetration into the alveolar space. An inflammatory response associated with eosinophilic infiltration follows, and the sequence of events that occurs in the lungs results in pneumonitis. The larvae migrate up the pulmonary tree, into the larynogpharynx, where they are swallowed (see the following image) and finally enter the intestine.
Stage 3
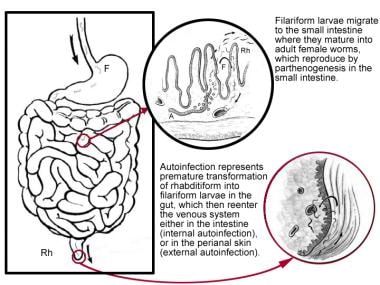
When filariform larvae reach the small bowel, they molt twice and mature into adult females (2 mm × 0.05 mm in diameter). The parasitic females produce eggs via parthenogenesis. Each adult female may live up to 5 years, in the intestinal folds, and continue the reproductive cycle. Strongyloides is the only helminth to secrete larvae (and not eggs) in feces. Their eggs hatch into noninfective rhabditiform larvae within the intestine. Larvae typically appear in feces approximately 1 month (about 28 days) after skin penetration, but the incubation period is unknown. The larvae are passed through the stool into the environment, where they mature into adult males and females (see the image below). As long as the patient is infected, which can be for several decades, the infection is communicable. The excreted rhabditiform larvae may again live freely in soil or be transformed into filariform larvae awaiting another human host. Alternatively, they may cause autoinfection.
Autoinfection
Autoinfection involves premature transformation of noninfective larvae (rhabditiform, 0.25 mm × 0.015 mm) into infective larvae (filariform, 0.5 mm × 0.015 mm), which can penetrate the intestinal mucosa (internal autoinfection) or the skin of the perineal area (external autoinfection), thus establishing a developmental (parasitic) cycle within the host. Infection can be maintained by repeated migratory cycles for the remainder of the host’s life.
Millions of filariform larvae reach the skin by means of the circulation or direct invasion from body cavities; they can migrate through all levels of the dermis and involve the subcutaneous tissue. The infective filariform larvae reenter the circulation by one of three methods: (1) The larvae penetrate the mucosa of the colon and cause indirect endoautoinfection; (2) the larvae penetrate the mucosa of the upper small intestine and cause direct endoautoinfection; or (3) the larvae penetrate the perianal skin and cause exoautoinfection. The last method has been associated with the development of larva currens.
After entering the circulation, the larvae are carried to the lungs, where the cycle repeats itself. This mechanism accounts for the chronicity and frequent recurrence of the disease in patients who no longer live in areas in which the disease is endemic.
Autoinfection is kept in check by a normal host immune response. However, in patients with impaired cell-mediated immunity, autoinfection may give rise to the two most severe forms of strongyloidiasis: hyperinfection syndrome (stage 4) and disseminated strongyloidiasis (stage 5). [1]
Stage 4
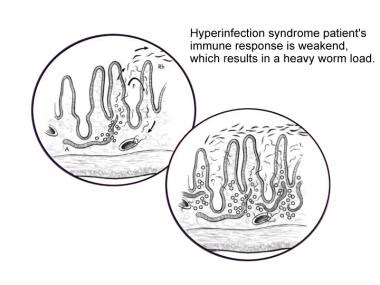
Hyperinfection syndrome represents an acceleration of the normal life cycle of S stercoralis, leading to excessive worm burden without the spread of larvae outside the usual migration pattern (eg, gastrointestinal tract, lungs) (see the following image). The larvae do not exit the host in feces and instead molt into the infective filariform larva within the intestinal lumen. These larvae are then capable of penetrating the bowel wall and traveling throughout the body. [1]
The pathophysiology that results from the hyperinfection cycle, which leads to dissemination in a compromised host, is not well understood. Patients receiving high-dose corticosteroids [6] or patients with human T-cell lymphotrophic virus type I (HTLV-I) are at particularly increased risk.
Stage 5
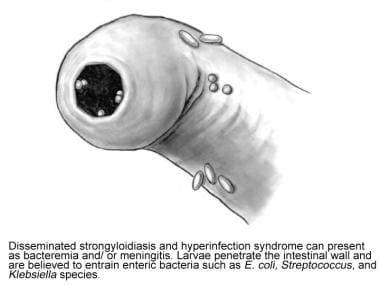
Disseminated strongyloidiasis involves widespread dissemination of larvae to extraintestinal organs (eg, central nervous system [CNS], heart, urinary tract, endocrine organs), which are outside the realm of the parasite's ordinary life cycle (see the image below). All organs and tissues may be invaded, along with the small intestine. In these severe forms, translocation of enteric bacteria may occur, leading to polymicrobial bacteremia and occasionally meningitis with enteric pathogens. The enteric pathogens may be carried on the filariform larvae or may enter the circulation through intestinal ulcers. The CNS, liver, and lungs are the most common destinations of the autoinfectious larvae.
Etiology
Strongyloidiasis is caused by the nematode (roundworm) Strongyloides stercoralis. The genus Strongyloides is classified in the order Rhabditida, and most members are soil-dwelling microbiverous nematodes. Most of the 52 species of Strongyloides do not infect humans. S stercoralis is the most common human pathogen. Other species include S myopotami and S procyonis. These species have animal hosts and are thus responsible for zoonotic infections. [7]
Infections are initiated when exposed skin contacts contaminate soil. Autoinfection commonly occurs, allowing infection to persist for decades. The longest documented asymptomatic infection was more than 65 years. Hyperinfection is typically triggered by drug-induced or disease-associated defects in cellular immunity, which allows a massive increase in parasite burden and dissemination to nearly all organ systems.
Risk factors for severe strongyloidiasis include the following:
-
Human T-cell leukemia virus type 1 (HTLV-1) infection [9, 10] : HTLV-1, the retrovirus associated with adult T-cell leukemia, has a bidirectional relationship with Strongyloides; coinfection with Strongyloides shortens the preleukemic phase of HTLV-1 infection [11] ; the Strongyloides antigen accelerates leukemogenesis, and treatment of the infection may actually decrease HTLV-1 viral load
-
Hypogammaglobulinemia
-
Malignancy/neoplasms, particularly hematologic malignancies (lymphoma, leukemia): Studies have suggested that Strongyloides infection may be associated with increased incidence of gastrointestinal lymphoma [13]
-
Malabsorption states and malnutrition
-
Chronic renal failure and end-stage renal disease
-
Diabetes mellitus
-
Advanced age
-
Collagen-vascular disease
-
Chronic alcohol consumption [4]
No evidence exists of direct person-to-person transmission in a household. Strongyloides larvae have been detected in the milk of mothers with chronic infection, suggesting vertical transmission. Evidence in dogs also shows transmission in breast milk. [18] No studies indicate transmammary transmission in humans exists. Cadaveric donors of renal transplants have been implicated as sources of fatal hyperinfection syndromes in transplant recipients. [19]
Zoonotic infections by Strongyloides species are similarly contracted by contact with sand or soil that contains infected animal droppings, including feces from raccoons and nutria. Infections are reported among veterinarians and laboratory workers who work in temperate climates and are exposed to larvae from horses. Zoonotic forms of Strongyloides infection can also produce creeping skin eruptions identical to those of S stercoralis infection.
Epidemiology
United States statistics
The true prevalence of S stercoralis is likely underestimated, because infection often is subclinical. Strongyloidiasis is uncommon in the United States, although endemic foci exist in rural areas of the southeastern states and the Appalachia region (especially in eastern Tennessee, Kentucky, and West Virginia) and Puerto Rico, [20] with prevalence rates close to 4%. Populations in whom strongyloidiasis is more prevalent include patients in long-term institutionalized care (mental health facilities, prisons), immigrants or refugees from tropical and subtropical countries (eg, Southeast Asia, Africa, Middle East), [21] and persons who were stationed in Southeast Asia during World War II [22] and the Vietnam War.
In one study, Southeast Asian immigrants living in Washington, DC, were found to have a 38% incidence of infection. [23] Similarly, a Canadian epidemiology study of Southeast Asian immigrants to Canada demonstrated infection in 11.8% in the Vietnamese population and a 76.6% seroprevalence in Cambodian immigrants. [24] Sudanese Lost Boys and Girls and Somali Bantu refugees demonstrated 46% and 23% respective seropositive rate. [25] Five percent of Vietnam veterans reporting mild symptoms had S stercoralis infection.
Infections acquired in the United States, while not usually associated with larva currens, are not clinically silent; the infected individuals usually have a chronic relapsing illness of mild to moderate severity. Among veterans of the US military forces who served in Southeast Asia, the prevalence of larva currens in those with confirmed strongyloidiasis is high, with studies showing a range of 30% to 90%. [26, 27]
International statistics
In 2017, the estimated strongyloidiasis prevalence was 8.1%, which corresponds to 613.9 million people infected with Strongyloides. [28] The global prevalence is ten times higher than previous estimates, ranging between 30 to 100 million people. The highest number of infected people live in the South-East Asia Region. Globally, prevalence rates of strongyloidiasis are as high as 40% in certain areas where suitably moist soil and improper disposal of human waste coexist, especially West Africa, the Caribbean, and Southeast Asia, as well as Colombia, tropical sections of Brazil, Panama, Cuba, and temperate sections of Spain. [29, 30]
The international prevalence of larva currens among patients with strongyloidiasis varies, with rates in the range of 30% to 90% in Southeast Asia. High rates of larva currens are also reported in Latin America. A stool and serosurvey for S stercoralis conducted in a community in the Peruvian Amazon region found strongyloidiasis due to S stercoralis in the stool of 69 (8.7%) of 792 participants. [31]
Racial and age differences in incidence
No racial predilection is apparent for Strongyloides infection; however, Southeast Asia appears to have the highest endemic percentage, and it is highly prevalent in some tropical Aboriginal communities in Australia. [32] Larva currens is most frequently seen in white patients with an infection acquired in Southeast Asia.
Although Strongyloides infection is represented in all ages, infection may most frequently initially occur in childhood, as children are most likely to play outdoors in contaminated soil with bare feet. [33] Advanced age is also a risk factor for severe strongyloidiasis, because it may be associated with an immunosuppressed state.
Prognosis
Acute and chronic strongyloidiasis carry a good prognosis in immunocompetent hosts. However, untreated infection can persist for the remainder of the patient's life because of autoinfection. Most immunocompetent individuals who develop strongyloidiasis have asymptomatic chronic infections that result in negligible morbidity. A patient's prolonged absence from an endemic area is no guarantee of freedom from infection.
Severe strongyloidiasis (hyperinfection syndrome and disseminated strongyloidiasis) carries a high mortality rate (up to 80%) because the diagnosis often is delayed. This relates to its nonspecific presentation and the host's immunocompromised status. Death from Strongyloides infection is typically iatrogenic and frequently occurs after an asymptomatic infected person is treated with immunosuppression. [34] Gram-negative sepsis is a common consequence of hyperinfection and carries a 50% mortality rate. [35] Disseminated strongyloidiasis may be relatively common in high-risk populations and may be frequently misdiagnosed as isolated gram-negative sepsis or acute respiratory distress syndrome.
(See Complications under Clinical.)
-
First stage, life cycle of Strongyloides stercoralis. Illustration by Tessa Kalman.
-
Second stage, life cycle of Strongyloides stercoralis. Illustration by Tessa Kalman.
-
Third stage, life cycle of Strongyloides stercoralis. Illustration by Tessa Kalman.
-
Fourth stage, life cycle of Strongyloides stercoralis. Illustration by Tessa Kalman.
-
Fifth stage, life cycle of Strongyloides stercoralis. Illustration by Tessa Kalman.
-
Rhabditiform larva of Strongyloides stercoralis in stool specimen (wet mount stained with iodine).