Background
Cat scratch disease (CSD), also known as cat scratch fever or subacute regional lymphadenitis, is a bacterial infection affecting lymph nodes that drain the sites of inoculation. Bartonella henselae, a gram-negative rod, is considered the principal etiologic agent. [1, 2] CSD is one of the most common causes of chronic lymphadenopathy in children and adolescents.
Patients with CSD usually have a history of sustaining a scratch or bite from a cat (typically a kitten). The initial symptom is formation of a papule at the inoculation site, followed by solitary or regional lymphadenopathy within 1-2 weeks. In most patients, the disease resolves spontaneously within 2-4 months.
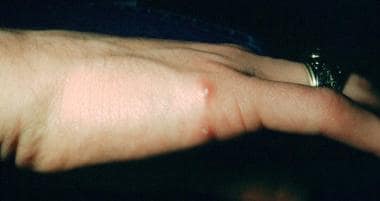 Papulopustular lesions of a primary inoculation site on the hand of a 16-year-old patient. These lesions had been present for approximately 3 weeks. A cat scratch antigen skin test was positive with 15-mm induration. No treatment was administered, and her condition resolved spontaneously in 2.5 months. Courtesy of Andrew Margileth, MD.
Papulopustular lesions of a primary inoculation site on the hand of a 16-year-old patient. These lesions had been present for approximately 3 weeks. A cat scratch antigen skin test was positive with 15-mm induration. No treatment was administered, and her condition resolved spontaneously in 2.5 months. Courtesy of Andrew Margileth, MD.
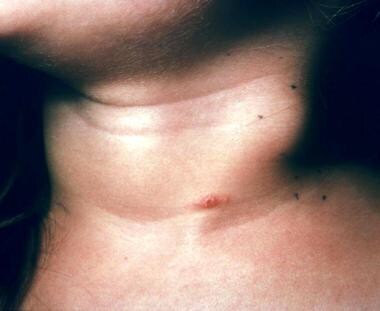 A crusted primary inoculation papule on the neck of a 4-year-old child. Note the adjacent lymphadenitis. This patient had contact with cats and had multiple scratches. Courtesy of Andrew Margileth, MD.
A crusted primary inoculation papule on the neck of a 4-year-old child. Note the adjacent lymphadenitis. This patient had contact with cats and had multiple scratches. Courtesy of Andrew Margileth, MD.
 This 13-year-old girl developed fatigue and malaise after being licked and scratched by a cat. The typical conjunctival granuloma was accompanied by a parotid mass and intraparotid adenitis. No treatment was administered, and all her signs and symptoms resolved in 3 months. Courtesy of Andrew Margileth, MD.
This 13-year-old girl developed fatigue and malaise after being licked and scratched by a cat. The typical conjunctival granuloma was accompanied by a parotid mass and intraparotid adenitis. No treatment was administered, and all her signs and symptoms resolved in 3 months. Courtesy of Andrew Margileth, MD.
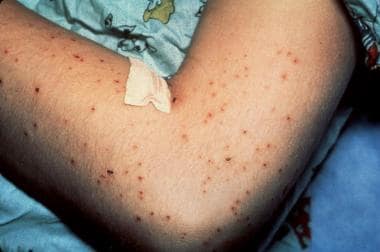 This 9-year-old boy developed cat scratch disease (CSD) encephalitis and a papular pruritic dermatitis after sustaining cat scratches and developing regional lymphadenitis. He was in a coma for 4 days, but experienced a complete and rapid recovery within 3 weeks. Biopsy of the skin rash revealed nonspecific changes. The CSD antigen skin test result was positive. Courtesy of Andrew Margileth, MD.
This 9-year-old boy developed cat scratch disease (CSD) encephalitis and a papular pruritic dermatitis after sustaining cat scratches and developing regional lymphadenitis. He was in a coma for 4 days, but experienced a complete and rapid recovery within 3 weeks. Biopsy of the skin rash revealed nonspecific changes. The CSD antigen skin test result was positive. Courtesy of Andrew Margileth, MD.
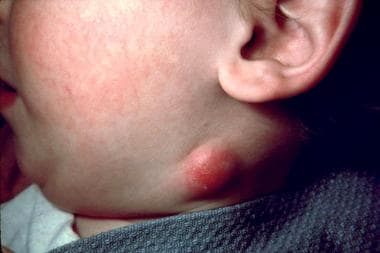 This 2.5-year-old boy was recovering from cat scratch disease acquired 10 months before when he developed this neck abscess over a period of 3 weeks. Biopsy revealed caseating granulomas; acid-fast bacillus and Warthin-Starry stain results were negative. Courtesy of Andrew Margileth, MD.
This 2.5-year-old boy was recovering from cat scratch disease acquired 10 months before when he developed this neck abscess over a period of 3 weeks. Biopsy revealed caseating granulomas; acid-fast bacillus and Warthin-Starry stain results were negative. Courtesy of Andrew Margileth, MD.
A small percentage of immunocompetent patients develop severe systemic disease or other atypical manifestations. These may include oculoglandular syndrome, encephalitis, neuroretinitis, pneumonia, osteomyelitis, erythema nodosum, arthralgia, arthritis, and thrombocytopenic purpura. [3, 4, 5, 6, 7, 8, 9, 10, 11, 12]
Infection of immunocompromised patients with the same organism leads to a very different disease, bacillary angiomatosis-peliosis. This disease is characterized by angioproliferative lesions resembling those of Kaposi sarcoma in the skin, liver, spleen, bone, and other organs.
It is necessary to diagnose CSD in a patient with lymphadenopathy to differentiate this benign process from a neoplastic process. [13] However, this can be difficult because of limitations to the currently available confirmatory diagnostic tests. [14]
Historical background
The history of CSD has been reviewed comprehensively by Carithers [15] in 1970 and by Margileth [16] in 1987. It is summarized here.
Henri Parinaud sometimes is given credit for the first description of CSD in 1889 [17] ; however, the oculoglandular syndrome of conjunctivitis with an enlarged preauricular lymph node that he reported ultimately was shown to comprise only a small subset of the possible clinical presentations of CSD, the result of inoculation of the CSD agent into the conjunctivae. Parinaud did not make the association with cat exposure; thus, his contribution is of limited scope.
In 1931, Dr. Robert Debré and his colleague Georges Semelaigne observed an unusual case of suppurating epitrochlear adenitis in a 10-year-old boy at the University of Paris and noted a number of cat scratches on the affected side. These were believed to be portals of entry for tubercle bacilli. When the tuberculin skin test results turned out to be negative, the investigators pursued an infectious cause of feline origin.
While bacteriologic investigations yielded no clues, the physicians continued to observe similar cases of spontaneously remitting regional lymphadenitis associated with cat scratches in their pediatric population. Debre postulated tularemia, pasteurellosis, infectious mononucleosis, or tuberculosis as possible etiologic agents, but with no convincing proof.
Foshay, a microbiologist at the University of Cincinnati, suspected CSD to be a possible manifestation of tularemia. On meeting Debre in 1947, the two investigators compared notes on "cat scratch disease" (Debre) and "cat fever" (Foshay). Foshay had produced an antigen from the pus of affected patients and achieved what was believed to be a diagnostic reaction after intradermal injection. Debre and his colleagues subsequently developed a similar antigen and demonstrated reactions in both old and new cases of CSD.
These results were presented and published in 1950. [18] These investigators also recorded failure of transmission of CSD to 15 different species of animals and possible human-to-human transmission in one of four cases.
In 1951, Greer and Keefer published the first report of CSD in American literature, in which they described a broader spectrum of CSD manifestations. [19] In the late 1950s, William Warwick of the University of Minnesota collaborated with Robert Good in an attempt to transmit CSD to "every variety of lab animal from the monkey to the mouse." Their only positive result was the development of cutaneous lymphadenopathy in a monkey given intracerebral injections of ground lymph nodes and pus.
In 1967, Carithers published the first thorough review of the world's literature on CSD, which included 567 references and detailed the manifold clinical presentations. This landmark publication of a series of 1200 cases evaluated by a single observer put the varied clinical presentations into perspective and provided the first realistic analysis of the spectrum of disease. [3]
Discovery and classification of the etiologic agent for CSD is one of the triumphs of modern microbiology. The elegance and power of molecular taxonomy applied to the CSD agent revealed unexpected connections with other well-recognized infectious diseases and a deeper understanding of the pathogenesis of CSD.
Both viruses and Chlamydia had been proposed as possible etiologic agents for CSD, until a small gram-negative motile coccobacillus was observed in infected lymphatic tissue using a Warthin-Starry stain and Brown-Hopp tissue Gram stain in 1983 at the Armed Forces Institute of Pathology. [20] In 1984, Margileth et al, using the same staining technique, demonstrated identical organisms in biopsy specimens taken from CSD inoculation papules. [21]
The first successful isolation and culture of the CSD organism was performed by English et al in 1988. [22] Their further studies fulfilled Koch's postulates, and the organism was determined to be the cause of CSD.
One of the isolates from the study by English et al was investigated at the US Centers for Disease Control and Prevention, along with additional specimens from Tripler Army Medical Center in Honolulu. From these specimens, the CSD organism was determined to be a new entity and given the name Afipia (from Armed Forces Institute of Pathology) felis.
Reports associating another agent (Rochalimaea henselae) with CSD began appearing in 1992. Although they are not closely related, R henselae and A felis are both members of the alpha-2 subclass of Proteobacteria and share a similar microscopic appearance and affinity for the Warthin-Starry stain.
R henselae already had been implicated in the pathogenesis of bacillary angiomatosis, an angioproliferative condition observed in patients who are immunocompromised. Reports of R henselae –associated CSD appeared, and new immunological data subsequently supported a major role for R henselae as the etiologic agent in CSD. Although R henselae now is believed to be the principal pathogen in CSD, both organisms have been reported in some patients with CSD. [23]
When the sequences of 16S bacterial rRNA from R henselae and Bartonella were compared, these organisms were determined to be so clearly closely related that they belonged in the same genus. Because Bartonella had historical precedence, R henselae was renamed Bartonella henselae.
A Medscape General Medicine article that may be of interest is " Do Bartonella Infections Cause Agitation, Panic Disorder, and Treatment-Resistant Depression? "
Pathophysiology
Most cases of CSD are caused by Bartonella henselae.Bartonella species are small pleomorphic, fastidious, facultative, gram-negative, and intracellular bacilli. Infection appears to confer lifelong immunity, as reports of recurrences of clinical cat scratch disease are rare. [24]
The hallmark of CSD is regional adenopathy proximal to the site of inoculation. In immunocompetent patients, Bartonella infection causes a granulomatous and suppurative response. In immunocompromised patients, the response to Bartonella infection can be vasculoproliferative with neovascularization. Bartonella is able to promote angioproliferation through adhesin A, which is observed in bacillary angiomatosis, peliosis, and verruga peruana.
Nine outer membrane proteins (OMP) of B henselae have been identified. The 43-kD OMP is a major protein capable of binding endothelial cells; further investigation is needed to clarify its role in the pathogenesis of CSD.
Feline infection with B henselae is common and asymptomatic. In the United States, 28% of surveyed cats had antibodies against the organism. In California, blood cultures were positive in 56% of domestic cats younger than 1 year and in 34% of cats older than 1 year. More than three fourths of all cats in California had antibodies to B henselae as evidence of prior infection; however, only 21% of pet cats were bacteremic, compared with 61% of stray cats.
A similar survey of cats in the Baltimore area found seropositivity in 12-14% of domestic cats versus 44% of feral animals. Cats can be asymptomatically bacteremic for months, even while antibody titers are developing.
The organism has been isolated from fleas residing on infected cats. Studies have shown that flea-vectored transmission of infection among cats occurs with high efficiency and that in the absence of fleas, infected cats do not transmit the infection to uninfected cats.
Although flea-vectored transmission to humans has not been documented, it could explain some cases in which the patient has no history of exposure to cats. Treatment of cats with doxycycline is associated with a reduction of bacteremia, but whether such treatment prevents relapse or reinfection is unknown.
Familial and household clustering of cases of CSD have been reported. However, only one member of a family in contact with an infected cat usually is affected.
Zangwill et al [25] found an 18% prevalence rate of seropositivity to B henselae among family members of patients with CSD. Upon further questioning, 43% of these individuals reported symptoms consistent with CSD during the previous 2 months. In the same study, matched control subjects not exposed to cats exhibited a 3.6% seropositivity rate. Carithers found similar results in a series of 1200 patients; 18.5% of asymptomatic family members had positive CSD antigen skin test results. [3]
An analysis of 605 healthy people in southern Spain found 13.55% IgG seropositive to B. henselae and to 11.07% B. quintana, delineating an elevated prevalence of both in this region. [26]
Many organ systems are affected by CSD, including the lymph nodes, CNS, eyes (neuroretinitis), skin (bacillary angiomatosis, erythema nodosum, erythema multiforme), lungs, and bones (arthritis and osteomyelitis).
Lymph nodes
In general, lymph nodes become enlarged in the 1-2 weeks after exposure. They often are tender and occasionally become fluctuant.
Lymphoid hyperplasia with arteriolar proliferation and reticular cell hyperplasia is seen early in the disease. As the disease progresses, granulomas appear, with central necrosis surrounded by lymphocytes. Histiocytes and multinucleated giant cells are often present. Finally, stellate microabscesses form, and nodes can become fluctuant.
Central nervous system
Encephalopathy is the most common neurologic manifestation, occurring in 2-3% of patients. This complication may be more common in adults than in children. The onset usually is abrupt and occurs 1-6 weeks after the lymphadenopathy becomes apparent.
Patients can become confused and disoriented, and their condition can deteriorate to coma. About 50% of patients have a fever. Focal findings of hemiparesis and reflex abnormalities may be noted. Seizures, which occur in as many as 80% of patients with neurologic sequelae, often are prolonged and recurrent.
The pathogenesis of encephalopathy is unknown, but it likely is not due to direct infection, because CSF usually is normal and recovery is rapid, often without antibiotic therapy. CT scans often are normal, and CSF examination shows mononuclear pleocytosis in 20-30% of patients. Electroencephalographs (EEGs) show nonspecific slowing. Recovery is usually complete in a 1 week or longer, but persistent neurologic deficits have been reported.
Neuroretinitis
Patients with neuroretinitis generally present with painless, unilateral visual loss. [27] Examination reveals decreased visual acuity, decreased color vision, and centrocecal scotoma. The optic disc appears edematous, and exudates frequently surround the macula.
Neuroretinitis is possibly due to a subretinal angiomatous nodule similar to that seen in bacillary angiomatosis.
Bacillary angiomatosis
Bacillary angiomatosis almost exclusively occurs in patients who are immunocompromised. Skin lesions consisting of numerous brown to violaceous or colorless vascular tumors of the skin and the subcutaneous tissue are the most common manifestation. Disseminated disease may involve bone, liver, spleen, lymph nodes, the gastrointestinal and respiratory tracts, and bone marrow.
B henselae and Bartonella quintana have been isolated from samples of cutaneous and osseous bacillary angiomatosis lesions. Histologic examination with Warthin-Starry staining reveals vascular proliferation with numerous bacillary organisms.
Pulmonary
Six cases of CSD with pneumonia and eight cases with pleural thickening and/or effusion have been reported. In these cases, pulmonary features developed 1-5 weeks after lymphadenopathy occurred. Systemic signs of infection, including fever, were present in 85%. One case in which a massive abscess involved the chest wall has been reported.
Vertebral osteomyelitis and splenic abscess
Rolain et al have described vertebral osteomyelitis with splenic abscess in a patient with CSD. [28] B henselae was specifically identified as the etiologic agent using several direct and indirect methods, including histologic and serologic assays, as well as immunofluorescent detection on slide appositions using a monoclonal antibody. This represents the second case of osteomyelitis associated with CSD.
Etiology
Cat scratch disease usually is caused by B henselae, formerly known as Rochalimaea henselae. B henselae is a small, fastidious, slow-growing, gram-negative, aerobic, nonmotile, pleomorphic bacillus. In the genus Bartonella, B bacilliformis, B quintana, B elizabethae, B vinsonii, and B koehlerae also are responsible for human disease. B clarridgeiae, rarely has been associated with cases of CSD.
Studies have demonstrated B henselae seropositivity rates ranging from 3.1-61.6% in the general population, depending on the country in which the study was performed. In all instances, few patients ever experienced symptoms, suggesting that only a minority of exposures to B henselae result in CSD.
Domestic cats are the natural reservoir and vectors of B henselae. In cats, B henselae infection is asymptomatic. Fleas are believed to transmit the bacteria between cats, and seroprevalence in cats is highest in warm or humid climates, where prevalence of flea infestation among cats is higher. A Bartonella-enzootic cycle involving wild cat species endemic in South Africa has been documented in southern Brazil. [29] Indirect immunofluorescence assay showed that 28% of serum samples of wild cats were seropositive for B henselae by immunofluorescence.
The transmission of B henselae from cats to humans occurs via a scratch or bite when the bacterium is present on the cat’s claws or oral cavity. More than 90% of patients with CSD have a recent history of contact with a cat, usually a kitten, and about 75% of these patients give a history of cat scratch or bite. Dogs have been implicated in 5% of cases. [30, 31] Occasional cases of infection associated with monkey bites also have been reported.
Kittens younger than 12 months are 15 times more likely to transmit the disease than adult cats. Kittens are more likely to be bacteremic with B henselae and are more likely to scratch. Individuals who have been scratched or bitten by a kitten are 27 times more likely to become infected, and people who have at least one kitten with fleas are 29 times more likely to become infected than people whose animals were free of fleas.
In approximately 1% of diagnosed cases, no animal scratch is implicated. Person-to-person transmission has not been documented. No evidence exists to suggest transmission from cat fleas to humans. Ticks and biting flies have also recently been recognized as potential vectors. [32] Cases occurring after scratches from thorns, wood splinters, and crab claws have occurred. However, in each of these cases, the patient recalled that a cat licked the abrasion.
Familial outbreaks have been documented and usually have involved siblings, or in rare instances parents. Symptoms among siblings often appear within 3 weeks of the index case.
A rare report describes a veterinarian who developed fever of unknown origin and persistent backache a month after an accidental needle puncture. Serology and molecular identification confirmed it to be B henselae infection. [33]
Epidemiology
United States statistics
A limited survey performed in 1993 by the Centers for Disease Control and Prevention reported approximately 22,000 cases of cat scratch disease (CSD) diagnosed annually, although many additional cases likely are unrecognized. [34] More than 2,000 hospital admissions with a discharge diagnosis of CSD are reported annually. [34] The estimated incidence among ambulatory patients is approximately 9.3 cases per 100,000 persons per year. [34] In 2000, approximately 437 pediatric hospitalizations associated with CSD were reported. [35]
Cases of CSD occur throughout the United States. The incidence is greater in regions with higher temperatures and humidity (eg, Hawaii, Pacific Northwest, southeastern states, coastal California), whereas Alaska, the Rocky Mountains, and midwestern states have a prevalence lower than the median. Only one genotype of B henselae has been reported in North America.
Approximately 70-90% of CSD cases occur in the fall and early winter months. This seasonality is presumed to be due to a midsummer rise in kitten births accompanied by increased flea infestation.
Reynolds et al found that the CSD-associated hospitalization rate in the United States was 0.60 per 100,000 patients younger than 18 years and 0.86 per 100,000 patients younger than 5 years. [35] The median hospitalization charge for cat scratch disease has been estimated to be $46,140, and annual expenses are estimated to be about $3.5 million.
International statistics
CSD has been reported worldwide; however, the international incidence is unknown. Seroprevalence rates vary greatly throughout the world, ranging from 0.6-37% and reflecting cat populations in each country. The disease is more prevalent in areas with warm humid climates.
In temperate climates, cat scratch disease predominantly occurs in autumn and winter; in the tropics, seasonal changes in frequency of the disease are not observed. Sanguinetti-Morelli et al (2011) found that in France, most cases of CSD occurred from September to April, with a peak in April. [36]
At least two genotypes of B henselae have been isolated from cats in Europe. B henselae is endemic in Europe, Africa, Australia, and Japan. In Germany, B henselae was the causative agent of head and neck lymphadenopathy in 61 (13.4%) of 454 patients [37] and the most common cause of lymphadenopathy in adults and children. [13, 37]
Sexual differences in incidence
In some case series, CSD occurs more frequently in males than females (male-to-female ratio of 3:2), whereas others show equal rates between males and females. One hypothesis to explain a greater incidence among males is the tendency toward rougher play with kittens and cats and consequently an increased risk for bites and scratches.
Age-related differences in incidence
In a database analysis by Jackson et al, 55% of patients with CSD were aged 18 years or younger. [34] Older literature suggests that more than 80% of cases occur in persons younger than 21 years.
The younger age of individuals most likely to acquire CSD reflects their likelihood of exposure to the major risk factor (ie, kittens). On the other hand, a bias may exist in the literature because pediatricians have collected many of the large case series.
Patients older than 60 years are more likely to present with atypical features of cat scratch disease. Especially in adults older than 49 years, CSD can occur concurrently with neoplasm and mycobacteriosis. [13]
Prognosis
The prognosis for immunocompetent patients with CSD is excellent. Complete recovery without sequelae occurs in nearly all patients. Significant morbidity occurs in 5-10% of cases, usually because of involvement of the central or peripheral nervous system or because of multisystem disseminated disease. Even in patients with CNS involvement, however, recovery without neurologic sequelae within weeks to months can be expected. Death caused by CSD in patients who are immunocompetent is extremely rare. [38, 39]
Lymphadenitis usually resolves spontaneously over 2-4 months, but 1-2 years may be required. One episode of cat scratch disease confers lifelong immunity to children and adolescents. Recurrent lymphadenopathy 6-13 months after the initial diagnosis has been reported in 3 adults with CSD.
Host immune status may be pivotal in determining the clinical manifestations. [40] Immunocompromised patients may experience a dramatic and potentially life-threatening course of disease. However, with appropriate antibiotic use and management of complications, these patients also typically experience full resolution of disease.
Patient Education
Assure parents that the disease will resolve spontaneously and that an episode of CSD confers long-term immunity. Although rare, adults can have recurrent illnesses.
Teaching children how to handle pets gently and declawing pets may decrease the incidence of CSD. The animal is infectious for only a short period, so removal of the pet from the home is not recommended. Advise patients and caregivers to control fleas on kittens to prevent spread of B henselae infection among animals.
-
Papulopustular lesions of a primary inoculation site on the hand of a 16-year-old patient. These lesions had been present for approximately 3 weeks. A cat scratch antigen skin test was positive with 15-mm induration. No treatment was administered, and her condition resolved spontaneously in 2.5 months. Courtesy of Andrew Margileth, MD.
-
A crusted primary inoculation papule on the neck of a 4-year-old child. Note the adjacent lymphadenitis. This patient had contact with cats and had multiple scratches. Courtesy of Andrew Margileth, MD.
-
This 13-year-old girl developed fatigue and malaise after being licked and scratched by a cat. The typical conjunctival granuloma was accompanied by a parotid mass and intraparotid adenitis. No treatment was administered, and all her signs and symptoms resolved in 3 months. Courtesy of Andrew Margileth, MD.
-
This 9-year-old boy developed cat scratch disease (CSD) encephalitis and a papular pruritic dermatitis after sustaining cat scratches and developing regional lymphadenitis. He was in a coma for 4 days, but experienced a complete and rapid recovery within 3 weeks. Biopsy of the skin rash revealed nonspecific changes. The CSD antigen skin test result was positive. Courtesy of Andrew Margileth, MD.
-
This 2.5-year-old boy was recovering from cat scratch disease acquired 10 months before when he developed this neck abscess over a period of 3 weeks. Biopsy revealed caseating granulomas; acid-fast bacillus and Warthin-Starry stain results were negative. Courtesy of Andrew Margileth, MD.
-
This 10-year-old child had contact with dogs, but not cats. The impressive lymphadenitis had been present for 5 weeks and was not tender. Pathologic examination of a biopsy specimen of the lymph node revealed nonspecific changes. She had a positive cat scratch disease skin test result and negative purified protein derivative skin test results. Treatment with cephalexin was administered with a good response. Complete resolution occurred in 4.5 months. Courtesy of Andrew Margileth, MD.
-
Warthin-Starry stained sections of lymph node showing chains and clusters of organisms. Courtesy of Andrew Margileth, MD.









