Background
Mercury in any form is poisonous, with mercury toxicity most commonly affecting the neurologic, gastrointestinal (GI) and renal organ systems. Poisoning can result from mercury vapor inhalation, mercury ingestion, mercury injection, and absorption of mercury through the skin. (See Etiology and Prognosis.)
Mercury has 3 forms: (1) elemental mercury, (2) inorganic salts, and (3) organic compounds. Perhaps the most deadly form of mercury is methylmercury. Only 2–10% of the ingested mercury is absorbed from the gut, and ingested elemental mercury is not absorbed at all; however, 90% of any methylmercury ingested is absorbed into the bloodstream from the GI tract. (See the images below.)
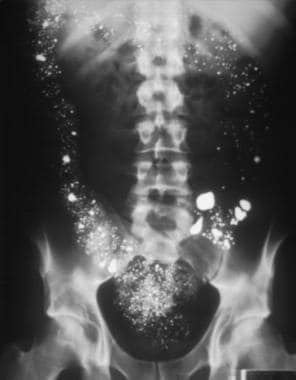 This is a 1-view, abdominal, upright radiograph in a male patient who intentionally ingested 8 ounces of elemental mercury. Notice how the mercury outlines the large intestine from ascending to descending. Image courtesy of Fred P. Harchelroad, MD, and Ferdinando L. Mirarchi, DO.
This is a 1-view, abdominal, upright radiograph in a male patient who intentionally ingested 8 ounces of elemental mercury. Notice how the mercury outlines the large intestine from ascending to descending. Image courtesy of Fred P. Harchelroad, MD, and Ferdinando L. Mirarchi, DO.
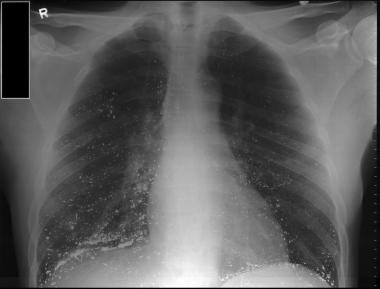 Patient with intentional ingestion of mercury from blood pressure instrument. Note how mercury beads can be seen deposited in lung fields. Image courtesy of Shuchi Vyas, MD.
Patient with intentional ingestion of mercury from blood pressure instrument. Note how mercury beads can be seen deposited in lung fields. Image courtesy of Shuchi Vyas, MD.
Organic mercury compounds, specifically methylmercury, are concentrated in the food chain. Fish from contaminated waters are the most common culprits. Industrial mercury pollution is often in the inorganic form, but aquatic organisms and vegetation in waterways such as rivers, lakes, and bays convert it to deadly methylmercury. Fish eat contaminated vegetation, and the mercury becomes biomagnified in the fish. Fish protein binds more than 90% of the consumed methylmercury so tightly that even the most vigorous cooking methods (eg, deep-frying, boiling, baking, pan-frying) cannot remove it. (See Etiology.)
For centuries, mercury was an essential part of many different medicines, such as diuretics, antibacterial agents, antiseptics, and laxatives. In the late 18th century, antisyphilitic agents contained mercury. It was during the 1800s that the phrase "mad as a hatter" was coined, owing to the effects of chronic mercury exposure in the hat-making industry, where the metal was used in the manufacturing process.
In 1889, Charcot, in his Clinical Lectures on Diseases of the Nervous System, attributed some rapid oscillatory tremors to mercury exposure. [1]
In Wilson's classic textbook of neurology, published in 1940, Wilson concurred with Charcot's attribution of tremors to mercury poisoning, but also described mercury-induced cognitive impairments, such as inattention, excitement, and hallucinosis. [2]
In 1961, researchers in Japan correlated elevated urinary mercury levels with the features of the previously mysterious Minamata disease. Before the etiology of Minamata disease was discovered, it plagued the residents around Minamata Bay in Japan with tremors, sensory loss, ataxia, and visual field constriction. (See Presentation.) [3]
Minamata disease is an example of organic toxicity. In Minamata Bay, a factory discharged inorganic mercury into the water. The mercury was methylated by bacteria and subsequently ingested by fish. Local villagers ate the fish and began to exhibit signs of neurologic damage, such as visual loss, extremity numbness, hearing loss, and ataxia. Babies exposed to the methylmercury in utero were the most severely affected. Furthermore, because mercury was also discovered in the breast milk of the mothers, the babies' exposure continued after birth. [4]
On January 19, 2013, The Minamata Convention on Mercury was agreed upon at the fifth session of the Intergovernmental Negotiating Committee in Geneva, Switzerland. It is a global treaty to protect human health and the environment from the adverse effects of mercury. The major highlights of the convention included a ban on new mercury mines, the phase-out of existing ones, control measures on air emissions, and the international regulation of the informal sector for artisanal and small-scale gold mining. [4] Treaty amendments in 2019 included restrictions on mercury-containing amalgams in dentistry.
Mercury is still found in many industries, including in battery, thermometer, and barometer manufacturing. Mercury can also be found in fungicides used in the agricultural industry. Before 1990, paints contained mercury as an antimildew agent. In medicine, mercury is used in dental amalgams and various antiseptic agents. (See Etiology and Prognosis.)
Mercury may also be contained in some cosmetics, such as skin-lightening products. One study measured international skin-lightening products for their mercury content, focusing on products available to US consumers either online or in stores. The products were screened for mercury content using a portable x-ray fluorescence spectrometer. Of the 549 products tested, 6% contained mercury levels above 1000 ppm, and 45% of the samples with mercury contained levels exceeding 10,000 ppm. Of lightening products purchased in the United States, 3.3% were found to contain mercury in excess of 1000 ppm. According to the authors, the Food and Drug Administration limits the amount of mercury in most cosmetic products to 1 ppm. [5]
Etiology
Organic methylmercury toxicity and inorganic mercury toxicity show different pathologic effects. Organic methylmercury toxicity causes prominent neuronal loss and gliosis in the calcarine and parietal cortices and cerebellar folia, as seen in cases of classic Minamata disease. [6]
Inorganic mercury causes cerebral infarctions, as well as systemic features, such as pneumonia, renal cortical necrosis, and disseminated intravascular coagulopathy. A more diffuse, direct neuronal toxicity may also exist with organic mercury, as the brain weights of patients with Minamata disease are substantially lower than those of controls. [7]
Nevertheless, both types of exposure may blur. In monkey models of methylmercury intoxication, demethylation resulted in inorganic mercury deposition in brain cells. [8]
Elemental mercury
Elemental mercury (Hg) is found in liquid form, which easily vaporizes at room temperature and is well absorbed (80%) through inhalation. Its lipid-soluble property allows for easy passage through the alveoli into the bloodstream and red blood cells (RBCs). Once inhaled, elemental mercury is mostly converted to an inorganic divalent or mercuric form by catalase in the erythrocytes. This inorganic form has similar properties to inorganic mercury (eg, poor lipid solubility, limited permeability to the blood-brain barrier, and excretion in feces). Small amounts of nonoxidized elemental mercury continue to persist and account for central nervous system toxicity.
Elemental mercury as a vapor has the ability to penetrate the central nervous system (CNS), where it is ionized and trapped, causing its significant toxic effects. Elemental mercury is not well absorbed by the GI tract; therefore, when it is ingested (eg, thermometers), it is only mildly toxic.
Inorganic mercury
Inorganic mercury toxicity occurs in several forms: metallic mercury (Hg), mercurous mercury (Hg1+), or mercuric mercury (Hg2+). Found mostly in the mercuric salt form (eg, batteries), inorganic mercury is highly toxic and corrosive. It gains access to the body orally or dermally and is absorbed at a rate of 10% of that ingested. It has a nonuniform mode of distribution secondary to poor lipid solubility and accumulates mostly in the kidney, causing significant renal damage. Although its poor lipid-solubility characteristics limit CNS penetration, slow elimination and chronic exposure allow for significant CNS accumulation of mercuric ions and subsequent toxicity. Long-term dermal exposure to inorganic mercury may also lead to toxicity.
Excretion of inorganic mercury, as with organic mercury, is mostly through feces. Renal excretion of mercury is considered insufficient and contributes to its chronic exposure and accumulation within the brain, causing CNS effects.
Organic mercury
Organic mercury can be found in 3 forms: aryl and short- and long-chain alkyl compounds. Organic mercurials are absorbed more completely from the GI tract than inorganic salts are; this is because of intrinsic properties, such as lipid solubility and mild corrosiveness (although organic mercury is much less corrosive than inorganic mercury).
Once absorbed, the aryl and long-chain alkyl compounds are converted to their inorganic forms and possess similar toxic properties to inorganic mercury. The short-chain alkyl mercurials (methylmercury) are readily absorbed in the GI tract (90–95%) and remain stable in their initial forms. Alkyl organic mercury has high lipid solubility and is distributed uniformly throughout the body, accumulating in the brain, kidney, liver, hair, and skin. Organic mercurials also cross the blood-brain barrier and placenta and penetrate erythrocytes, producing neurologic symptoms, teratogenic effects, and high blood to plasma ratio, respectively.
Nervous system effects
Methyl mercury exerts its most devastating effect on the CNS by causing psychiatric disturbances, ataxia, visual loss, hearing loss, and neuropathy. Neurologic damage in the form of diffuse and widespread neuronal atrophy is most severe in patients exposed in utero.
Excretion of alkyl mercury occurs mostly in the form of feces (90%), secondary to significant enterohepatic circulation. The biological half-life of methylmercury is approximately 65 days.
Mercury damages the nervous system through several potential mechanisms. Mercury binds to sulfhydryl groups and incapacitates key enzymes involved in the cellular stress response, protein repair, and oxidative damage prevention. [9] Methylmercury disrupts the muscarinic cholinergic systems in the brainstem and occipital cortices as well. [10]
Methylmercury also inactivates sodium-potassium adenosine triphosphatase (Na+/K+-ATPase), which leads to membrane depolarization, calcium entry, and eventual cell death. [11] Several pathways may be simultaneously activated converging in apoptosis. [12]
Researchers have also identified excessive excitotoxins and dysregulation of the nitric oxide system in rodents exposed to methylmercury. [13] Methylmercury can also induce brain edema, and this may produce sulcal artery compression and consequent ischemia, which may account, at least in part, for the calcarine and parietal cell loss and gliosis.
Furthermore, methylmercury may sequester the element selenium and thereby disrupt cellular biochemical pathways that use selenium as an enzymatic cofactor. In addition, selenium-mercury binding itself may sequester and protect against mercury toxicity. [14] Indeed, substantial evidence suggests that supplementing selenium ameliorates or even reverses mercury toxicity in laboratory animals. [15]
The effects of mercury may also be modified by a person’s genetic milieu. For example, the interaction between mercury exposure and a genetic polymorphism in heme biosynthesis (coproporphyrinogen oxidase) yielded additive impairments on a test of visual-motor skills in dental workers, [16] and, more recently, additive impairments were documented between urinary mercury levels and a serotonin transporter polymorphism on motor control tasks in a similar population. [17] Furthermore, certain heat shock protein polymorphisms have been associated with symptomatic mercury toxicity compared with asymptomatic, but, similarly exposed, controls, [18] in particular single-nucleotide polymorphisms of metallothionein (a group of heavy metal-binding proteins), have been associated with reduced hair mercury levels among dental professionals. [19]
Finally, glutathione conjugates with methylmercury and facilitates excretion in the gastrointestinal tract. A polymorphism of the glutathione transferease gene has been associated with elevated blood mercury levels among Jamaican children. [20]
Renal effects
Necrosis of the proximal tubules is a common direct renal toxic effect. Unexplained renal abnormalities with neuropsychiatric disturbances should prompt the physician to consider mercury toxicity.
Sources of mercury poisoning
Causes of elemental mercury toxicity include the following:
-
Thermometers
-
Barometers
-
Batteries
-
Bronzing
-
Calibration instruments
-
Chlor-alkali production
-
Dental amalgams
-
Electroplating
-
Ethnomedical practices
-
Fingerprinting products
-
Fluorescent and mercury lamps
-
Infrared detectors
-
Jewelry industry
-
Manometers
-
Neon lamps
-
Paints
-
Paper pulp production
-
Photography
-
Silver and gold production
-
Semiconductor cells
In the United States, exposure to organic mercury is primarily through ingestion of contaminated fish. Persons who consume large amounts of seafood from contaminated waters have an increased risk of toxicity. Surveys indicate that public awareness of the risks of mercury-contaminated fish is limited.
The causes of organic mercury toxicity also include the following:
-
Antiseptics
-
Bactericidals
-
Embalming agents
-
Farming industry
-
Fungicides
-
Germicidal agents
-
Insecticidal products
-
Laundry products
-
Diaper products
-
Paper manufacturing
-
Pathology products
-
Histology products
-
Seed preservation
-
Wood preservatives
The causes of inorganic mercury toxicity include the following:
-
Antisyphilitic agents
-
Acetaldehyde production
-
Chemical laboratory work
-
Cosmetics
-
Disinfectants
-
Explosives
-
Embalming
-
Fur hat processing
-
Ink manufacturing
-
Mercury vapor lamps
-
Mirror silvering
-
Perfume industry
-
Photography
-
Spermicidal jellies
-
Tattooing inks
-
Taxidermy production
-
Vinyl chloride production
-
Wood preservation
Newer compact, energy-efficient fluorescent lights contain substantial mercury concentrations, making breakages with subsequent release a concerning source of exposure. [21]
Products from mercury cell chlor-akali plants used in the production of high-fructose corn syrup have been shown to contain detectable levels of mercury. [22]
Mercury-containing disk batteries are a concern because of their ability to cause corrosion and ulceration of the GI mucosa. With battery ingestion, one would expect signs of inorganic mercury exposure, such as hypersalivation and vomiting, rather than signs of organic mercury poisoning.
One major risk factor for mercury toxicity is industrial contamination. Workers employed in the manufacturing of mirrors, thermometers, fluorescent lights, and radiography machines, as well as in gold mining, are at risk for inorganic mercury poisoning. Organic mercury poisoning can occur among exposed workers in the paper and pulp industries.
New York City public health officials traced potentially toxic urinary mercury levels in residents to contaminated skin-lightening creams, which were subsequently removed from stores and embargoed. [23] Identifying these sources of exposure may be difficult in the clinic, as patients may be embrassed to discuss such skin-lightening treatments. [24] . Others have identified renal disease secondary to mercury toxicity from such putative beautifying topicals. [25]
Traditional religious and healing practices are risk factors for mercury exposure. Mercury has been identified as a contaminant in Chinese herbal balls, [26] and it has been used in the Santeria religion, as well as in Tibetan medicine. [27] Indeed, spectrophotometric measurements of mercury vapor concentrations were elevated in New Jersey buildings located near "botanicas" in a primarily Latino community, compared with a control community. [28] Furthermore, of herbal Ayurvedic preparations, 20% were found to contain high levels of mercury. [29, 30]
Even the mercury vapors from dental amalgam have been of concern as a possible, although controversial, source of exposure among dental workers and the general population. A study of 1663 veterans used a wide battery of noncognitive tests and found no clinically evident deficits associated with amalgam exposure. However, a subclinical decrement in vibration as measured by an automated device correlated with amalgam exposure and accounted for 15% of the variance in a multiple regression model. [31, 32] Other studies have found no consistent correlation between urinary mercury levels and nerve conduction parameters among dental professionals. [33]
Two randomized studies of a total of 1041 children aged 6–10 years whose dental caries were treated with either amalgam or resin composite fillings showed no group differences on extensive batteries of neuropsychological tests after 5–7 years of follow-up. [34, 35] After an exhaustive investigation and review of the evidence, including the form of mercury in question, the route of exposure, and the dose, the Public Health Service concluded that dental amalgams do not pose a serious health risk. [32, 36] In 2020, the American Dental Association (AD) reaffirmed amalgam's safety. [37]
Intentional self-poisoning with oral or injected inorganic mercury has been described, with outcomes varying from coma and death despite heroic efforts [38] to surprisingly scant clinical sequelae in the setting of persistently elevated blood mercury levels 5 years after the attempt. [39]
A very controversial source of organic mercury exposure is thimerosal, a preservative used in vaccines to prevent bacterial contamination. Thimersol was removed from childhood vaccines in the United States in 2001. No definite link between this small amount of mercury and any known disease has been found. Nonetheless, concerns over mercury content in vaccines has led to the increased availability of mercury-free ones. [40, 41, 42]
Epidemiology
Occurrence in the United States
The 2022 Annual Report of the American Association of Poison Control Centers' National Poison Data System documented 1143 single exposures to mercury or compounds containing mercury. Overall, 30 individuals suffered moderate toxcity, 5 had severe toxicity, and none died as a result of mercury exposure. [43]
From 1999 to 2016, organic blood mercury levels increased in US children and pregnant women, while inorganic mercury levels declined among the overall population. [44]
International occurrence
Worldwide, outbreaks of methylmercury intoxication are sporadic. Minamata Bay in Japan was involved in the first and most famous epidemic, but not the largest. In the early 1970s, one of the most severe mass poisonings in history occurred in Iraq, when nearly 95,000 tons of seed grains treated with a methylmercury-based fungicide were accidentally baked into bread for human consumption. [45] More than 6000 individuals were hospitalized, and hundreds died. Many were hospitalized for weeks before methylmercury intoxication was correctly diagnosed.
Mercury mining areas in China have also contributed to cases of methylmercury poisoning through the ingestion of rice grown in contaminated soil. [46] Small-scale gold mining in developing countries has also produced mercury toxicity. [47]
Age-related demographics
Toxicity probably affects developing fetuses and children preferentially compared with other age groups, but even on this point, the data are incomplete. Prenatal exposure through maternal consumption of predominantly fish and whale meat has been shown to impair development among children in the Faroe Islands, while maternal mercury exposure from fish consumption alone in the Seychelles did not result in significant developmental problems among children prenatally exposed. [48, 49] The protective effects of the naturally selenium-enriched fish diet of the Seychelles population has been proposed to explain this effect. [15]
In a case of a family exposed to methylmercury through the ingestion of contaminated pork, the more severe clinical manifestations were found in the younger children. [50]
Prognosis
Recovery from mercury poisoning is variable. Acute fulminant intoxication with methylmercury resulted in coma and death in the Minamata catastrophe. Delayed toxicity can also occur, as in a fatal case in which symptoms developed only several months following absorption of dimethylmercury through the skin. [51] Mercury vapors also can result in acute neurologic and generalized symptoms.
While the cognitive and emotional sequelae of mercury exposure, at least in adults, may diminish with time, tremors and neuropathic changes have been reported to persist for decades after inorganic mercury exposure. For example, among 104 workers examined 30 years following inorganic mercury exposure, the presence of resting tremors correlated significantly with prior cumulative mercury exposure. [52, 53]
Outcome in mercury toxicity depends on the form of the mercury compound and the severity of exposure. Mild exposure to inorganic (ie, elemental, mercuric salt) and organic compounds can result in a complete recovery. Fatality is usually the result of severe exposure to mercuric salt. Most significant organic mercury exposures leave a neurologic sequela. Minimal dermal exposure to dimethylmercury has resulted in progressive neurologic deterioration and death, with initial symptoms delayed for several months.
In a large Chinese study of 288 patients with chronic, mainly inorganic mercury toxicity, most patients with neuropathy recovered within 3 years; the outlook for renal toxicity was less clear because many patients were lost to follow up. [54]
All forms of mercury are toxic to a fetus, but methylmercury most readily passes through the placenta. Even with an asymptomatic patient, maternal exposure can lead to spontaneous abortion or retardation.
Individuals who need to be admitted to the hospital include the following:
-
Individuals who ingested (or are thought to have ingested) mercury salts
-
Individuals thought to have elemental mercury inhalation and have pulmonary injury
-
Individuals who require intensive chelating therapy
Minamata disease
Once the neurologic sequelae of Minamata disease are evident, the damage is irreversible, and severe intoxications have been fatal. However, the damage may be minimized if detected early enough. Effects of long-term exposure are only now being fully recognized. Most survivors of Minamata disease have chronic neuropathologic conditions such as the following:
-
Ataxia
-
Visual-field loss
-
Psychiatric disturbances
-
Sensory loss
-
Chronic paresthesias
Compared with other patients, babies exposed to Minamata disease in utero have a more dismal prognosis. Their sequelae include the following:
-
Severe developmental delay
-
Low birth weight
-
Persistent cognitive impairment
Of the original 121 individuals from Minamata Bay who were affected, nearly one third died shortly after their initial presentation. Subsequent investigations since the late 20th century resulted in the identification of more than 2000 additional patients who were affected by chronic sequelae of Minamata disease. Continued cognitive impairments decades after initial exposure have been identified even in those moderately exposed, as measured by umbilical cord mercury. [55]
Dietary consumption of mercury
The primary source of environmental exposure to mercury in the general population is through the consumption of contaminated fish. [56] Fish consumption has clear health benefits, and the risk posed by mercury exposure is currently speculative. The fetal brain is more susceptible to mercury-induced damage than that of an adult. As a result of this data, the Environmental Protection Agency (EPA) reduced the allowable intake of methylmercury from 0.5 mcg to 0.1 mcg of mercury per kilogram per day, which is lower than the amount allowable according to other regulatory agencies. [57]
The EPA guideline is derived from reports of subtle and small neuropsychological changes in children in a study in the Faeroe Islands; the children’s exposure was mainly from whale consumption. [58] A similar study in the Seychelles found no adverse effects from fish consumption alone. [59]
The Food and Drug Administration (FDA) has recommended that pregnant women, breastfeeding mothers, and young children avoid eating fish with a high mercury content (>1 ppm), such as shark, swordfish, tilefish, and king mackerel. This also includes fresh and frozen tuna (mercury content between 0.5 ppm and 1.5 ppm). Canned tuna has exhibited variable mercury concentrations, with one study finding that 55% of cans contained mercury levels greater than 1 ppm, with white tuna demonstrating higher levels than light tuna. [60]
From a nonprofessional perspective, this translates into a weekly consumption of 1 can (198g, or 7oz) of tuna for an adult. [61] Rather than ban the sale of these species, Health Canada recommends that they be consumed no more than once per week or once per month by children and by women of childbearing age. [62] Mercury levels in freshwater fish vary, but, in general, bass, pike, muskellunge, and walleye have high levels of mercury and should be eaten in moderation. Provincial guidelines for sport fish often mirror federal seafood recommendations. [63]
Inhalation exposure
Acute exposure caused by inhaled elemental mercury can lead to pulmonary symptoms. Initial signs and symptoms, such as fever, chills, shortness of breath, metallic taste, and pleuritic chest pain. Other possible symptoms include stomatitis, lethargy, confusion, and vomiting. In addition, elemental mercury can also be injected, causing a life-threatening pulmonary embolism.
Recovery is usually without sequela, but pulmonary complications of inhaled toxicity may include interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, and interstitial fibrosis. Fatal acute respiratory distress syndrome (ARDS) has been reported following elemental mercury inhalation.
Vaccine-associated exposure
Thimerosal is a mercury-containing preservative used in some vaccines and other products since the 1930s. No harmful effects have been reported from thimerosal at doses used in vaccines, except for minor reactions, such as redness and swelling at the injection site. However, in July 1999, the Public Health Service agencies, the American Academy of Pediatrics, and vaccine manufacturers agreed that thimerosal should be reduced or eliminated in vaccines as a precautionary measure. Today, with the exception of some influenza vaccines, none of the vaccines used in the United States to protect preschool children against 12 infectious diseases contain thimerosal as a preservative. [64]
Exposure to mercury has been suggested to contribute to the development of autism in children. Although the mechanism for this disorder has many hypotheses, no evidence has confirmed a causal relationship between mercury exposure and the development of autism. In fact, in one study, the discontinuation of thimerosal-containing vaccines in Denmark seemed to be followed by an increase in incidence of autism. [65]
In 2004, Immunization Safety Review Committee of the US Institute of Medicine (IOM) shifted from the position of neutrality to the conclusion that "the evidence favors rejection of a causal relationship between thimerosal-containing vaccines and autism." [66] Since 2004, 2 cohort studies from the United Kingdom examined the relationship between thimerosal contained within vaccines and autism, and their conclusions were in agreement with the IOM that there is no causal relationship between the two.
Patient Education
Unfortunately, a clinician can do little to prevent mercury toxicity. Public education can raise awareness about the risks associated with easily preventable sources of toxicity, such as contaminated herbal preparations or mercury thermometers.
Publicizing the amount of mercury contained in frequently eaten fish can help to reduce toxicity on a local level.
Mercury is found often in household items such as fluorescent bulbs and the risk of potential exposure has increased. The need to understand proper procedure for disposing of potential exposure is critical. The EPA has outlined a clear process to dispose of accidental spillage. For complete details on mercury spills with specifics related to a broken thermometer or fluorescent bulb, visit the EPA Web site. [67]
Lax occupational safeguards and protocol adherence continue to contribute to serious exposures. For example, recently workers in a fluorescent light recycling plant with poor safety practices exhibited elevated urinary mercury levels and neurologic symptoms. [68]
Minamata disease typically occurs in areas in which the population depends on seafood as a dietary staple and in areas in which industrial wastes contaminate the drinking water. Educate patients about alternative food sources and about eliminating their intake of contaminated fish.
Outbreaks of methylmercury poisoning also have occurred after the introduction of fungicide-treated grain into the food supply. Neither humans nor livestock should eat seed grain treated with mercurial fungicides.
-
This is a 1-view, abdominal, upright radiograph in a male patient who intentionally ingested 8 ounces of elemental mercury. Notice how the mercury outlines the large intestine from ascending to descending. Image courtesy of Fred P. Harchelroad, MD, and Ferdinando L. Mirarchi, DO.
-
Patient with intentional ingestion of mercury from blood pressure instrument. Note how mercury beads can be seen deposited in lung fields. Image courtesy of Shuchi Vyas, MD.









