Practice Essentials
Tuberculosis (TB) (see the image below), a multisystemic disease with myriad presentations and manifestations, is the most common cause of infectious disease–related mortality worldwide. After falling steadily since at least 2012, TB cases began to climb in 2020 and rose 5% in 2022 to 8,300 cases.
Although TB rates are decreasing in the United States, the disease is becoming more common in many parts of the world. In addition, the prevalence of drug-resistant TB is increasing worldwide.
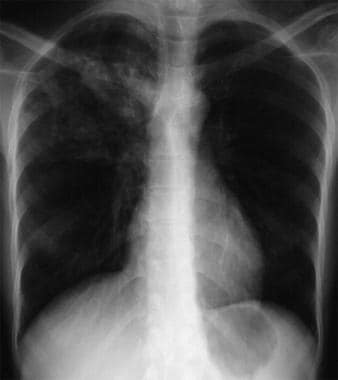 Anteroposterior chest radiograph of a young patient who presented to the emergency department (ED) with cough and malaise. The radiograph shows a classic posterior segment right upper lobe density consistent with active tuberculosis. This woman was admitted to isolation and started empirically on a 4-drug regimen in the ED. Tuberculosis was confirmed on sputum testing. Image courtesy of Remote Medicine (remotemedicine.org).
Anteroposterior chest radiograph of a young patient who presented to the emergency department (ED) with cough and malaise. The radiograph shows a classic posterior segment right upper lobe density consistent with active tuberculosis. This woman was admitted to isolation and started empirically on a 4-drug regimen in the ED. Tuberculosis was confirmed on sputum testing. Image courtesy of Remote Medicine (remotemedicine.org).
To help identify and manage infectious travel diseases, see Travel Medicine and Vaccination and Critical Images slideshow Travel Diseases to Consider Before and After the Trip.
Signs and symptoms
Classic clinical features associated with active pulmonary TB are as follows (elderly individuals with TB may not display typical signs and symptoms):
-
Cough
-
Weight loss/anorexia
-
Fever
-
Night sweats
-
Hemoptysis
-
Chest pain (also can result from tuberculous acute pericarditis)
-
Fatigue
Symptoms of tuberculous meningitis may include the following [1] :
-
Headache that either has been intermittent or persistent for 2-3 weeks
-
Subtle mental status changes that may progress to coma over days to weeks
-
Low-grade or absent fever
Symptoms of skeletal TB may include the following:
-
Back pain or stiffness
-
Lower-extremity paralysis in as many as half of patients with undiagnosed Pott disease
-
Tuberculous arthritis, usually involving only 1 joint (most often the hip or knee, followed by the ankle, elbow, wrist, and shoulder)
Symptoms of genitourinary TB may include the following [2] :
-
Flank pain
-
Dysuria
-
Frequent urination
-
In men, a painful scrotal mass, prostatitis, orchitis, or epididymitis
-
In women, symptoms mimicking pelvic inflammatory disease
Symptoms of gastrointestinal TB are referable to the infected site and may include the following [3] :
-
Nonhealing ulcers of the mouth or anus
-
Difficulty swallowing (with esophageal disease)
-
Abdominal pain mimicking peptic ulcer disease (with gastric or duodenal infection)
-
Malabsorption (with infection of the small intestine)
-
Pain, diarrhea, or hematochezia (with infection of the colon)
Physical examination findings associated with TB depend on the organs involved. Patients with pulmonary TB may have the following:
-
Abnormal breath sounds, especially over the upper lobes or involved areas
-
Rales or bronchial breath signs indicating lung consolidation
Signs of extrapulmonary TB differ according to the tissues involved and may include the following [4] :
-
Confusion
-
Coma
-
Neurologic deficit
-
Lymphadenopathy
-
Cutaneous lesions
The absence of any significant physical findings does not exclude active TB. Classic symptoms often are absent in high-risk patients, particularly those who are immunocompromised or elderly.
See Clinical Presentation for more details.
Diagnosis
Screening methods for TB include the following:
-
In vitro, blood test based on interferon-gamma release assay (IGRA) with antigens specific for Mycobacterium tuberculosis for latent infection
Obtain the following laboratory tests for patients with suspected TB:
-
Acid-fast bacilli (AFB) smear and culture using sputum obtained from the patient. Absence of a positive smear result does not exclude active TB infection; AFB culture is the most specific test for TB
-
HIV serology in all patients with TB and unknown HIV status: individuals infected with HIV are at increased risk for TB
Other diagnostic tests may warrant consideration, including the following:
-
Specific enzyme-linked immunospot (ELISpot)
-
Nucleic acid amplification tests
-
Blood culture
Positive cultures should be followed by drug susceptibility testing; symptoms and radiographic findings do not differentiate multidrug-resistant TB (MDR-TB) from fully susceptible TB. Such testing may include the following:
-
Direct DNA sequencing analysis
-
Automated molecular testing
-
Microscopic-observation drug susceptibility (MODS) and thin-layer agar (TLA) assays
-
Additional rapid tests (eg, BACTEC-460, ligase chain reaction, luciferase reporter assays, FASTPlaque TB-RIF)
Obtain a chest radiograph to evaluate for possible associated pulmonary findings. The following patterns may be seen:
-
Cavity formation: indicates advanced infection; associated with a high bacterial load
-
Noncalcified round infiltrates; may be confused with lung carcinoma
-
Homogeneously calcified nodules (usually 5-20 mm): tuberculomas, representing old infection
-
Primary TB: typically, pneumonialike picture of the infiltrative process in middle or lower lung regions
-
Reactivation TB: pulmonary lesions in the posterior segment of the right upper lobe, apicoposterior segment of the left upper lobe, and apical segments of the lower lobes
-
TB associated with HIV disease: frequently atypical lesions or normal chest radiographic findings
-
Healed and latent TB: dense pulmonary nodules in hilar or upper lobes; smaller nodules in upper lobes
-
Miliary TB: numerous small, nodular lesions that resemble millet seeds
-
Pleural TB: empyema may be present, with associated pleural effusions
Evaluation considerations for extrapulmonary TB include the following:
-
Biopsy of bone marrow, liver, or blood cultures
-
If tuberculous meningitis or tuberculoma is suspected, perform a lumbar puncture
-
If vertebral ( Pott disease) or brain involvement is suspected, CT or MRI is necessary
-
If genitourinary complaints are reported, urinalysis and urine cultures can be obtained
See Workup for more details.
Management
Physical measures (if possible or practical) include the following:
-
Isolate patients with possible TB in a private room with negative pressure
-
Have medical staff wear high-efficiency disposable masks sufficient to filter the bacillus
-
Continue isolation until sputum smears are negative for 3 consecutive determinations (usually after approximately 2-4 weeks of treatment)
Initial empiric pharmacologic therapy consists of the following 4-drug regimens:
Special considerations for drug therapy in pregnant individuals include the following:
-
Streptomycin should not be used
-
Preventive treatment is recommended during pregnancy
-
Pregnant individuals are at increased risk for isoniazid-induced hepatotoxicity
-
Breastfeeding can be continued during preventive therapy
Special considerations for drug therapy in children include the following:
-
Most children with TB can be treated with isoniazid and rifampin for 6 months, along with pyrazinamide for the first 2 months if the culture from the source case is fully susceptible.
-
For postnatal TB, the treatment duration may be increased to 9 or 12 months.
-
Ethambutol often is avoided in young children.
Special considerations for drug therapy in HIV-infected patients include the following:
-
Rifampin must be avoided in patients receiving protease inhibitors; rifabutin may be used instead
Considerations in patients receiving antiretroviral therapy include the following:
-
Patients with HIV and TB may develop a paradoxical response when starting antiretroviral therapy
-
Starting antiretroviral therapy early (eg, < 4 weeks after the start of TB treatment) may reduce progression to AIDS and death [8]
-
In patients with higher CD4+ T-cell counts, it may be reasonable to defer antiretroviral therapy until the continuation phase of TB treatment [9]
Multidrug-resistant TB
Multidrug-resistant TB (MDR-TB) refers to isolates that are resistant to both isoniazid and rifampin (and possibly other drugs). When MDR-TB is suspected, start treatment empirically before culture results become available; obtain molecular drug susceptibility testing, if possible. Modify the initial regimen, as necessary, based on susceptibility results. Never add a single new drug to a failing regimen. Administer at least 5 drugs for the intensive phase of treatment and at least 4 drugs for the continuation phase (listed in order of preference), as follows [10, 11] :
-
A fluoroquinolone: levofloxacin or moxifloxacin preferred
-
Clofazimine (available only through Investigational New Drug application through the FDA)
-
An aminoglycoside: streptomycin or amikacin preferred
-
Delamanid
-
Para-aminosalicylic acid
Surgical resection is recommended for patients with MDR-TB whose prognosis with medical treatment is poor. Procedures include the following:
-
Segmentectomy (rarely used)
-
Lobectomy
-
Pneumonectomy
-
Pleurectomy for thick pleural peel (rarely indicated)
Latent TB
Recommended regimens for isoniazid and rifampin for latent TB have been published by the US Centers for Disease Control and Prevention (CDC) [12, 13, 14] : An alternative regimen for latent TB is isoniazid plus rifapentine as self-administered or directly observed therapy (DOT) once-weekly for 12 weeks [15, 16] ; it is not recommended for children under 2 years, individuals who are pregnant or who plan to become pregnant, or patients with TB infection presumed to result from exposure to a person with TB that is resistant to 1 of the 2 drugs.
See Treatment and Medication for more details.
Background
Tuberculosis (TB), a multisystemic disease with myriad presentations and manifestations, is the second most common cause of infectious disease–related mortality worldwide after COVID-19. The World Health Organization (WHO) has estimated that 2 billion people have latent TB and that globally, in 2021, the disease killed 1.6 million people, including 187,000 people with HIV. [17] (See Epidemiology.)
After falling steadily since 1992, TB cases began to climb in 2020 and rose 5% in 2022 to 8,300 cases. The disease is becoming more common in many parts of the world, and the prevalence of drug-resistant TB also is increasing worldwide. Coinfection with the human immunodeficiency virus (HIV) has been an important factor in the emergence and spread of resistance. [18] (See Treatment.)
Mycobacterium tuberculosis (Mtb), a tubercle bacillus, is the causative agent of TB. It belongs to a group of closely related organisms in the M tuberculosis complex, including M africanum, M bovis, and M microti.(See Etiology.) An image of the mycobacterium is seen below.
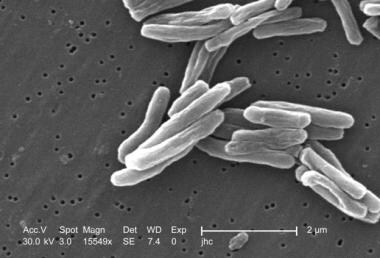 Under a high magnification of 15549x, this scanning electron micrograph depicts some of the ultrastructural details seen in the cell wall configuration of a number of Gram-positive Mycobacterium tuberculosis bacteria. As an obligate aerobic organism, M. tuberculosis can only survive in an environment containing oxygen. This bacterium ranges in length between 2-4 microns, with a width between 0.2-0.5 microns. Image courtesy of the Centers for Disease Control and Prevention/Dr. Ray Butler.
Under a high magnification of 15549x, this scanning electron micrograph depicts some of the ultrastructural details seen in the cell wall configuration of a number of Gram-positive Mycobacterium tuberculosis bacteria. As an obligate aerobic organism, M. tuberculosis can only survive in an environment containing oxygen. This bacterium ranges in length between 2-4 microns, with a width between 0.2-0.5 microns. Image courtesy of the Centers for Disease Control and Prevention/Dr. Ray Butler.
The lungs are the most common site for the development of TB; a majority of patients with TB present with pulmonary complaints. [19] Of 7174 TB cases reported in the United States in 2020, extrapulmonary TB (with no demonstrated pulmonary involvement) accounted for approximately 21% of cases. Disease in both pulmonary and extrapulmonary sites was reported in 78.9% of cases. Relative numbers are essentially unchanged since 2018, despite a 20% reduction in reported cases of TB in the United States in 2020 versus 2019. [20] Extrapulmonary TB can occur as part of a primary or late generalized infection.(See Pathophysiology and Presentation.)
The primary screening method for TB infection (active or latent) is the Mantoux tuberculin skin test with purified protein derivative (PPD). An in vitro blood test based on interferon-gamma release assay (IGRA) with antigens specific to M tuberculosis also can screen for latent TB infection. Patients suspected of having TB should submit sputum for acid-fast bacilli (AFB) smear and culture.(See Workup.)
The usual treatment regimen for TB cases from fully susceptible M tuberculosis isolates consists of 6 months of multidrug therapy. Empiric treatment starts with a 4-drug regimen of isoniazid, rifampin, pyrazinamide, and ethambutol or streptomycin; this therapy subsequently is adjusted according to susceptibility testing results and toxicity. Pregnant individuals, children, HIV-infected patients, and patients infected with drug-resistant strains require different regimens.(See Treatment and Medication.)
Laws vary from state to state, but infectious disease laws typically empower public health officials to investigate suspected cases of TB, including potential contacts of persons with TB. In addition, patients may be incarcerated for nonadherence to therapy. The role of public health/tuberculosis-trained nurses and digital adherence technologies increasingly is being explored and recognized, particularly in resource-limited settings. [21, 22]
New TB treatments are being developed, and new TB vaccines are under investigation.(See Epidemiology and Treatment.)
Historical background
TB is an ancient disease. Signs of skeletal TB (Pott disease) have been found in remains from Europe from Neolithic times (8000 BCE), ancient Egypt (1000 BCE), and the pre-Columbian New World. TB was recognized as a contagious disease by the time of Hippocrates (400 BCE) when it was termed "phthisis" (Greek from phthinein, to waste away). In English, pulmonary TB was long known by the term "consumption." The discovery of a stethoscope by Laennec in 1816 and x-rays in 1895 is credited to tuberculosis. [23, 24] Laennec's stethoscope was created to decrease the physical distance between physicians and patients to reduce the risk of transmission rather than amplifying sounds. His nephew, Mériadec Laennec, is said to have diagnosed tuberculosis in Laennec using Laennec's stethoscope. Later, on March 24, 1882, German physician Robert Koch identified and isolated M tuberculosis as the cause of tuberculosis. The observance of World Tuberculosis Day on March 24 commemorates Koch's discovery.
The worldwide incidence of TB increased with population density and urban development, so by the Industrial Revolution in Europe (1750), it was responsible for more than 25% of adult deaths. In the early 20th century, TB was the leading cause of death in the United States; during this period, however, the incidence of TB began to decline because of various factors, including the use of basic infection-control practices (eg, isolation).
Resurgence of TB
The US Centers for Disease Control and Prevention (CDC) has recorded detailed epidemiologic information on TB since 1953. Beginning in 1985, a resurgence of TB was noted with strains resistant to rifampin and isoniazid. The increase was observed primarily in ethnic minorities and especially in persons living with HIV. TB control programs were revamped and strengthened across the United States. The TB rates began to fall after 1992 due to the introduction of antiretroviral treatment (ART) and improvement in TB diagnosis, treatment, and prevention measures. An artificial decline in TB rate was noted in 2020 due to underdiagnosis and social distancing, followed by a rebound in 2021. [25] Cases rose to 8,300 in 2022. (See Epidemiology.)
TB is most common worldwide in Africa, the West Pacific, and Eastern Europe. These regions are plagued with factors that contribute to the spread of TB, including the presence of limited resources, HIV infection, and multidrug-resistant (MDR) TB. (See Epidemiology.) The coinfection rates of TB with HIV are highest in South Africa, Nigeria, and India. Persons living with HIV are 18 times more likely to develop active tuberculosis disease compared to people without HIV, and they are 3 times more likely to die during tuberculosis treatment as per the 2020 global TB report. [26]
Drug-resistant TB
Drug resistance was first noted in the 1940s when streptomycin was formally studied as monotherapy for the treatment of TB. In 1952, the availability of isoniazid made TB curable in most patients, and the addition of rifampin in 1970 allowed therapeutic interventions to utilize multidrug regimens to decrease the risk for drug resistance.
Despite combination therapy, outbreaks of multidrug-resistant TB (MDR-TB) occurred throughout the world in the mid-1990s, including the United States, Spain, Italy, Argentina, and Russia. [27, 28, 29, 30] Back then, the outbreak patterns mirrored the epidemiology of the HIV epidemics (especially in countries with the least infrastructure to screen and manage patients co-infected with TB and HIV), explaining why later in the late 1990s and early 2000, the number of TB cases in regions such as sub-Saharan Africa increased dramatically. [31]
Multidrug-resistant TB ( MDR-TB) is defined as resistance to isoniazid and rifampin, which are the 2 most effective first-line drugs for TB. Extensively drug-resistant TB (XDR TB) is a rare type of MDR TB that is, in addition to isoniazid and rifampin, resistant to any fluoroquinolone and at least one of three injectable second-line drugs (ie, amikacin, kanamycin, or capreomycin). XDR-TB resistant to all anti-TB drugs tested has been reported in Italy, Iran, and India and remains a risk factor for international cases due to travel and immigration. [32] Because XDR TB is resistant to the most potent TB drugs, patients are left with treatment options that are much less effective. XDR TB is of special concern for persons with HIV infection or other conditions that can weaken the immune system because the persons are more likely to develop TB disease once they are infected and also have a higher risk for death once they develop TB.
WHO has kept track of TB drug resistance rates since 1994. Worldwide, 3.3% of new TB cases and 20.1% of previously treated cases are MDR. It is estimated that 490,000 cases of MDR TB existed worldwide in 2016. Certain areas, such as Belarus, Latvia, Estonia, the Russian oblasts of Ivanovo and Tomsk, and the Henan Province of China, have high rates of MDR in newly diagnosed patients (exceeding 18%). [33]
Multiple factors contribute to the drug resistance of M tuberculosis, including incomplete and inadequate treatment or adherence to treatment, logistical issues, virulence of the organism, multidrug transporters, host genetic factors, and HIV infection. A study from South Africa found high genotypic diversity and geographic distribution of XDR-TB isolates, suggesting that acquisition of resistance, rather than transmission, accounts for between 63% and 75% of XDR-TB cases. [34]
Increased resistance rates could be explained at least partially by increased testing. In 2020, 71% (approximately 2.1 million out of 3.0 million people) with laboratory-confirmed pulmonary TB were tested for rifampicin resistance, up from 61% in 2019 and 50% in 2018. Among these 2.1 million cases, 157,903 (7.52%) had drug-resistant TB, including 25,681 cases of pre-XDR-TB or XDR-TB. [35]
In the United States, the rate of primary resistance to an antituberculosis drug has remained stable at about 12%, with that of MDR-TB at about 1%. [33] Of note, the COVID-19 pandemic was associated with a large proportional drop in people diagnosed with drug-resistant TB (201,997 in 2019), which mirrored the proportional drop in the number of new people diagnosed with any form of TB. This is thought to be due to the COVID-19 pandemic-related health systems disruptions and the decrease in community support systems, especially in areas endemic for TB. [36] These disruptions translated into the lack of diagnostic services in certain areas and the lack of linkage to treatment and care worldwide. [37]
The cure rate in persons with MDR-TB is 50-60%, compared with 95-97% for persons with drug-susceptible TB. [38] The estimated cure rate for XDR-TB is 30-50%. In people who also are infected with HIV, MDR-TB and XDR-TB often produce fulminant and fatal disease; the time from TB exposure to death averages 2-7 months. In addition, these cases are highly infectious, with conversion rates of as much as 50% in exposed health-care workers.
Global surveillance and treatment of TB
As previously stated, multidrug resistance has been driven by poor adherence to TB therapies, resulting in difficulties in controlling the disease. Consequently, a global pandemic threat occurred in the late 1980s and early 1990s. Reacting to these signals, the WHO developed a plan to try to identify 70% of the world's TB cases and completely treat at least 85% by 2000.
These goals led to the creation of major TB surveillance programs and the concept of directly observed therapy (DOT), which requires a third party to witness compliance with pharmacotherapy. Thanks to these efforts, the global detection of smear-positive cases rose from 11% (1991) to 45% (2003), with 71-89% of those cases undergoing complete treatment.
Approach to TB in the emergency department
Despite the importance of early isolation of patients with active TB, a standardized triage protocol with acceptable sensitivities has yet to be developed. [39] Moran et al demonstrated that among patients with active TB in the emergency department (ED), TB often was unsuspected, and isolation measures were not used. [40] The difficulty in establishing such a protocol only highlights the importance of the emergency physician's role in promptly identifying and isolating active TB.
A large percentage of ED patients are at increased risk of having active TB, including homeless/shelter-dwelling patients, travelers from endemic areas, immunocompromised patients, healthcare workers, and incarcerated patients. Therefore, emergency physicians must consider the management and treatment of TB as a critical public health measure in the prevention of a new epidemic. [41]
For high-risk cases, prehospital workers can help identify household contacts who also may be infected or at high risk of becoming infected.
Prehospital workers should be aware that any case of active TB in a young child indicates disease in one or more adults with close contact, usually within the same household. TB in a child is a sentinel event indicating recent transmission.
Extrapulmonary Involvement in TB
Extrapulmonary involvement occurs in one fifth of all TB cases; 60% of patients with extrapulmonary manifestations of TB have no evidence of pulmonary infection on chest radiographs or in sputum cultures. [4]
Cutaneous TB
The incidence of cutaneous TB appears low. Even in areas with high TB prevalence, such as India or China, cutaneous manifestations of TB (overt infection or the presence of tuberculids) have been found in less than 0.1% of individuals seen in dermatology clinics. Clinical manifestations may include patches and plaques (lupus vulgaris, TB verrucosa cutis), macules and papules (acute miliary TB, papulonecrotid tuberculid, lichen scrofulosorum), nodules, and abscesses (erythema induratum of Bazin, tuberculous gumma), erosions, and ulcers (tuberculous chancre, orificial TB, scrofuloderma) leading to delayed diagnosis and treatment. [42]
Ocular TB
TB can affect any structure in the eye and typically presents as a granulomatous process. [43] Ocular mycobacterial infection occurs in nonendemic areas and cannot be ruled out with negative chest imaging. [44] Keratitis, iridocyclitis, intermediate uveitis, retinitis, scleritis, and orbital abscesses are within the spectrum of ocular disease. Choroidal tubercles and choroiditis are the most common ocular presentations of TB. Adnexal or orbital disease may be seen with preauricular lymphadenopathy. Because of the wide variability in the disease process, presenting complaints will vary.
Most often, patients will complain of blurry vision that may or may not be associated with pain and red eye. Proptosis, double vision, or extraocular muscle motility restriction may be the presenting complaint in the rare case of orbital disease. Preseptal cellulitis in children with spontaneous draining fistula also may occur. There may be ocular findings without ocular complaints in both pulmonary and extrapulmonary TB cases.
Patient Education
Patient information on TB can be found at the following sites:
-
World Health Organization Tuberculosis
Pathophysiology
Infection with M tuberculosis (Mtb) results most commonly through exposure of the lungs or mucous membranes to infected aerosols. Droplets in these aerosols are 1-5 μm in diameter; in a person with active pulmonary TB, a single cough can generate 3000 infective droplets, with as few as 10 bacilli needed to initiate infection. [45] Exposure to a similar infectious dose can occur within 5 minutes of a conversation with an infected individual. The highest number of bacilli per mL are reported of sputum with laryngeal tuberculosis, making it a highly contagious variant. [46]
Mtb are highly antigenic and initially promote a vigorous, nonspecific immune response. Their antigenicity is due to multiple cell wall constituents, including glycoproteins, phospholipids, and wax D, which activate Langerhans cells, lymphocytes, and polymorphonuclear leukocytes. After the Mtb bacilli are inhaled, they enter resident pulmonary alveolar macrophages via endocytosis and disrupt phagosome maturation through multiple pathways, including the activation of the Early Secreted Target Antigen 6 Secretion System 1 (ESX1) secretion system, delaying the T cell responses via the proteasomal pathway of antigen presentation and secretion of mycobacterial urease, superoxide dismutase, catalase, thioredoxin, and other antioxidants that neutralize reactive oxygen species generated by phagocytes. [46, 47] The mycobacteria are released by macrophages and directly infect alveolar epithelial cells or transmigrate within infected macrophages through the lung parenchyma. The released Mtb antigens are processed by dendritic cells. The dendritic cells activate CD4+ (Th) cells through the IL-12 pathway in lymphatic tissue. The CD4+ cells then migrate to the site of infection and activate macrophages, leading to the accumulation of lytic enzymes and regulatory molecules, including tumor necrosis factor (TNF-Alpha) and transforming growth factor-beta, orchestrating granuloma formation. [48]
Granuloma formation is the hallmark of Mtb infection. It originally was thought to have a role in "walling-off" or containing the mycobacterium, but it increasingly is recognized to play a significant role in harboring mycobacterium in a metabolically dormant state with a 10% probability of reactivation in latently infected hosts. A granuloma consists of infected and uninfected macrophages, epitheliod cells (highly stimulated macrophages), multinucleated giant cells (Langhans giant cells), dendritic cells, monocytes, eosinophils, mast cells, B and T lymphocytes, neutrophils and nonhematopoietic cells including fibroblasts, and epithelial and endothelial cells around Mtb. [49] Molecular studies in murine models from the last 100 years have demonstrated a complex interplay that will tip the balance in favor of either the host (to contain the infection) or mycobacterium (to disseminate throughout the host). This intricate interplay is based on interactions between macrophages infected with mycobacterium, uninfected macrophages, and neutrophils at molecular levels. [50] Granulomas can have multiple phenotypes and can be fibrotic, calcified, suppurative, cellular, or non-cellular depending upon the predominant cell type and the presence or absence of Mtb; various forms can be seen in a single host at a given time. [51, 52, 53, 54]
The macrophages continue to be recruited and commit to granuloma formation through chemotactic pathways that depend on transcriptional induction of region of difference( RD1), ESX-1-dependent matrix metalloproteinase-9 (MMP-9), and early secretory antigenic target of T cells (ESAT-6). [49, 55, 56] The exact mechanisms by which ESX-1 induces MMP-9 and favors macrophage recruitment remains unknown, but this hypothesis is supported by increased MMP pleural fluid levels in patients with the granulomatous pleural disease compared to non-granulomatous disease. [57] Granuloma also serves as a vehicle for mycobacterial expansion through intracellular spread via the region of difference(RD1)/ESX1 virulence focus.
The 'mature' macrophages undergo either apoptosis or necrosis at the end of their lifecycle. During the apoptosis of mature macrophages, RD1/MMP9 signaling plays a role in chemo-attraction and recruitment of new macrophages. The new macrophages then engulf the apoptotic debris of previously infected macrophages and their mycobacterial contents. On the other hand, a foamy phenotype is induced in the macrophages by ESX-1 competent mycobacteria, leading to a switch in metabolism from glycolysis to ketosis and accumulation of lipids and fatty products within macrophages. The lipid-laden foamy macrophage contributes to the characteristic caseous necrosis and provides lipid nutrition to the mycobacterium it hosts. [58, 59, 60] This is followed by tissue necrosis at the site (terminus Ghon focus) and involvement of nearby lymph nodes and calcification (Ghon complex). The newly infected macrophages and dendritic cells can efflux from primary granulomas to establish secondary granulomas, thus aiding dissemination. [61, 62] The extracellular survival and dissemination of mycobacterium upon necrosis of phagocytes is supported by their cording morphology described by Koch in 1882, successfully preventing re-phagocytosis.
MMP-1 has been implicated in the degradation of the fibrous extracellular matrix surrounding the granuloma, eventually leading to cavitation. [63] A higher mycobacterial burden is described for cavitary lesions, which could contribute to the emergence of multi-drug resistance due to higher bacterial load and difficult drug penetration. [64, 65, 66, 67]
When a person is infected with Mtb, the innate/acquired immune system is activated, which can suppress Mtb infection into an inactive form called latent tuberculosis infection (LTBI). In a subgroup of individuals exposed to TB, the initial innate immune response and acquired T cell response (without priming/memory) can eliminate Mtb infection. [68] These individuals have been termed "resistors" and represent < 10% of the population. [46] These persons will have negative tuberculin skin tests (TST) and interferon-gamma release assay (IGRA). [69] These patients also will not benefit from LTBI treatment. Some individuals can eradicate the infection while retaining a strong memory T cell, which a positive TST or IGRA translates. This individual also will not benefit from LTBI treatment. Other individuals cannot eliminate the pathogen, so the mycobacterial infection will persist in a quiescent/latent stage that TST or IGRA can detect. However, these patients will benefit from LTBI treatment to prevent the progression to active disease.
Notably, subclinical TB is an increasingly recognized entity in which patients will intermittently have a positive mycobacterial culture (with a negative smear given the low bacilliary load) without exhibiting major clinical symptoms (they could be completely asymptomatic or have mild symptoms). [70] Patients with subclinical TB disease can be contagious and will benefit from multi-drug therapy (as is the case for active TB).
The lungs are the most common site for the development of TB, and the majority of patients with TB present with pulmonary complaints. However, extrapulmonary TB also can occur as part of a primary or late generalized infection. An extrapulmonary location also may serve as a reactivation site, and extrapulmonary reactivation may coexist with pulmonary reactivation.
The most common sites of extrapulmonary disease are as follows (the pathology of these lesions is similar to that of pulmonary lesions) [4] :
-
Mediastinal, retroperitoneal, and cervical (scrofula) lymph nodes - The most common site of tuberculous lymphadenitis (scrofula) is in the neck, along the sternocleidomastoid muscle; it usually is unilateral and causes little or no pain; advanced cases of tuberculous lymphadenitis may suppurate and form a draining sinus
-
Vertebral bodies
-
Adrenals
-
Meninges
-
GI tract
Infected end organs typically have high regional oxygen tension (eg, the kidneys, bones, meninges, eyes, choroids, and the apices of the lungs). M tuberculosis infection causes tissue destruction primarily by inciting intense host immune reactions to antigenic cell wall proteins.
Uveitis caused by TB is the local inflammatory manifestation of a previously acquired primary systemic tubercular infection. There is some debate about molecular mimicry and responses to noninfectious tubercular antigens, leading to active ocular inflammation without bacterial replication.
TB lesions
The typical TB lesion is an epithelioid granuloma with central caseation necrosis. The most common site of the primary lesion is within alveolar macrophages in subpleural lung regions. Bacilli proliferate locally and spread through the lymphatics to a hilar node, forming the Ghon complex.
Early tubercles are spherical, 0.5- to 3-mm nodules with 3 or 4 cellular zones demonstrating the following features:
-
A central caseation necrosis
-
An inner cellular zone of epithelioid macrophages and Langhans giant cells admixed with lymphocytes
-
An outer cellular zone of lymphocytes, plasma cells, and immature macrophages
-
A rim of fibrosis (in healing lesions)
Initial lesions may heal, and the infection may become latent before symptomatic disease occurs. Smaller tubercles may resolve completely. Fibrosis occurs when hydrolytic enzymes dissolve tubercles, and a fibrous capsule surrounds larger lesions. Such fibrocaseous nodules usually contain viable mycobacteria and are potential lifelong foci for reactivation or cavitation. Some nodules calcify or ossify and easily are seen on chest radiographs.
Tissues within areas of caseation necrosis have high levels of fatty acids, low pH, and low oxygen tension, all of which inhibit the growth of the tubercle bacillus.
Depending on the host immune response, lesions that develop around mycobacterial foci can be proliferative or exudative. Both lesions can develop in the same host since infective dose and local immunity vary from site to site.
Proliferative lesions develop when the bacillary load is small and the host cellular immune responses dominate. In these settings, the tubercles are compact and constitute activated macrophages, proliferating lymphocytes, plasma cells, and an outer rim of fibrosis. Intracellular killing of mycobacteria is effective, and the bacillary load remains low.
Exudative lesions predominate when large numbers of bacilli are present, and host defenses are weak. Thus, loose aggregates of immature macrophages, neutrophils, fibrin, and caseation necrosis and mycobacterial growth ensue. Without treatment, these lesions progress, and infection spreads.
On another note, if the host cannot control the initial infection, the patient develops progressive primary TB with tuberculous pneumonia in the lower and middle lobes of the lung. Purulent exudates with large numbers of acid-fast bacilli can be found in sputum and tissue, and subserosal granulomas may rupture into the pleural or pericardial spaces and create serious inflammation and effusions.
Pulmonary TB lesions:
Pulmonary TB mainly is acquired through inhalation. After Mtb enters the alveoli, it translocates to the lung parenchyma through the macrophages or the transendothelial pathway. Antigen-presenting cells activate the helper T cells (TH) and stimulate differentiation into TH1 cells through a complex juxtaposition of cytokines at the molecular level. TH1 cells produce interferon-gamma (INFɣ), which in turn activates macrophages. The simultaneous innate and cellular immunity recruitment leads to granuloma formation. Lipid-laden foamy macrophage contributes to the characteristic caseous necrosis and provides lipid nutrition to the mycobacterium it hosts. [58, 59, 60] The primary lesion can heal spontaneously with a calcified nodule (Ghon focus) left afterward. Mtb also can travel to regional lymph nodes (causing hilar and paratracheal lymphadenopathy) and other areas in the lung parenchyma, stimulating granuloma formation. The terminus Ghone focus and the calcified necrosis of the nearby lymph nodes form the Ghon complex.
The primary disease can progress in immunosuppressed individuals and children with cavitation, pleural effusions, and hematogenous spread (miliary disease).
Etiology
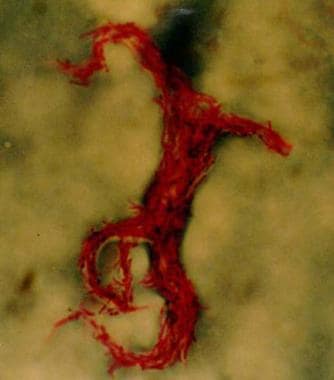
TB is caused by M tuberculosis (Mtb), a slow-growing obligate aerobe and a facultative intracellular parasite. The organism grows in parallel groups called cords (as seen in the image below).
Mtb are non-spore-forming and nonmotile curved intracellular rods measuring 0.2-0.5 μm by 2-4 μm. Their cell walls contain mycolic acid-rich, long-chain glycolipids and phosphoglycolipids (mycocides) that protect mycobacteria from cell lysosomal attack [71] . The mycolic acid component also helps retain red basic fuchsin dye after acid rinsing (acid-fast stain), the basis of the acid-fast stains used for pathologic identification.
Transmission
Humans are the only known reservoir for Mtb. The organism is spread primarily via airborne aerosol from an individual in the infectious stage of TB (although transdermal and GI transmission have been reported). TB also is reported to be transmitted through organ transplant, and screening protocols are in place with close surveillance of high-risk donors. Risk factors for developing active disease after Mtb infection include recent acquisition (within 18 months), exposure to higher infectious inoculum, malnutrition, tobacco smoking, pulmonary fibrotic lesions, alcoholism, intravenous drug use, and comorbidities, including HIV, diabetes, silicosis, immunosuppression (particularly TNF-alpha inhibitors), cancer of head and neck, hematological malignancies, end-stage renal disease, chronic malabsorption, and gastrectomy [46] .
Microepidemics have occurred in closed environments such as submarines and on transcontinental flights. Populations at high risk of acquiring the infection also include healthcare workers, inner-city residents, nursing and correctional facilities home residents.
Molecular typing of M tuberculosis isolates in the United States by restriction fragment-length polymorphism analysis suggests that more than one-third of new TB cases result from person-to-person transmission. The remainder results from the reactivation of latent infection.
Verhagen et al demonstrated that large clusters of TB are associated with increased tuberculin skin test–positive contacts, even after adjusting for other transmission risk factors for transmission. [72] The number of positive contacts was significantly lower for index cases with isoniazid-resistant TB than those with fully-susceptible TB. This suggests that some TB strains may be more transmissible than others and that isoniazid resistance is associated with lower transmissibility.
Extrapulmonary spread
Because Mtb can survive and proliferate within mononuclear phagocytes, which ingest the bacterium, Mtb can invade local lymph nodes and spread to extrapulmonary sites, such as the bone marrow, liver, spleen, kidneys, bones and joints, pleura, genitourinary tract, peritoneum, pericardium, meninges, and brain, usually via hematogenous routes.
Although mycobacteria are spread by blood throughout the body during initial infection, primary extrapulmonary disease is uncommon except in immunocompromised hosts. Extrapulmonary spread has been reported in up to 10-40% of patients with TB, with even higher rates in PLHIV. [46] Infants, older persons, or otherwise immunosuppressed hosts cannot control mycobacterial growth and can develop disseminated (primary miliary) TB. Patients who become immunocompromised months to years after the primary infection also can develop late, generalized disease.
Role of TNF antagonists and steroids
Tumor necrosis factor–-alpha (TNF-α) is produced by infected and activated macrophages and pro-inflammatory T cells. It enhances macrophage activation, chemokine production by macrophages, and immune cell recruitment during Mtb infection. Anti-TNF-α monoclonal antibody administration may result in the dissolution of intact granulomas, the release of viable mycobacteria, and disease reactivation. This can explain the higher incidence of TB observed in patients receiving anti-TNF-α treatment. Treatment with tumor necrosis factor–alpha (TNF-α) antagonists, which are used for rheumatoid arthritis, psoriasis, and several other autoimmune disorders, has been associated with a significantly increased risk for TB. [73] Reports have included atypical presentations, extrapulmonary and disseminated disease, and deaths. Patients scheduled to begin therapy with a TNF-α antagonist should be screened for latent TB and counseled regarding the risk for TB.
Immunosuppressive therapy includes long-term administration of systemic steroids (prednisone or its equivalent, given >15 mg/day for ≥4 wk or more) and inhaled steroids. Brassard and colleagues reported that inhaled steroids, in the absence of systemic steroids, were associated with a relative risk of 1.5 for TB. Inaheld corticosteroids at higher doses appear to increase the risk for TB. [74]
TB in children
In children younger than 5 years, the potential for the development of fatal miliary TB or meningeal TB is a significant concern. Osteoporosis, sclerosis, and bone involvement are more common in children than in adults with TB. The epiphyseal bones can be involved because of their high vascularity. Children do not commonly infect other children because they rarely develop a cough, and their sputum production is scant. However, cases of child-child and child-adult TB transmission are well documented. (See Pediatric Tuberculosis for complete information on this topic.)
Genetic factors
The genetics of tuberculosis are quite complex, involving many genes. The genetic predisposition might explain the mathematical probability of developing active disease in only a fraction of exposed individuals. Some genes involve important aspects of the immune system, whereas others involve more specific mechanisms by which the human body interacts with mycobacterium species. The following genes have polymorphisms associated with either susceptibility to or protection from tuberculosis. Additionally, regions such as 8q12 - q13 are associated with increased risk, although an exact mechanism or candidate gene has not been found.
Natural resistance-associated macrophage protein 1 (NRAMP1), CD14 and mannose-binding lectin (MBL)
Macrophages, lymphocytes, and lung parenchyma express the NRAMP1 gene. It is translated into an integral membrane protein expressed exclusively in the lysosomal compartment of monocytes and macrophages, where it can alter the intraphagosomal equilibrium to influence microbial replication. The human NRAMP1 has been renamed solute carrier family 11 (SLC11-A1). [75]
A report from Africa described an association of 4 different polymorphisms of the NRAMP1 gene with an increased risk for TB. Subjects with 2 of those 4 polymorphisms (located in an intron and a region upstream from the coding region) were at particular risk of contracting TB. [76] The association of NRAMP1 with the risk for TB has been replicated in subsequent studies. [77, 78, 79, 80]
MBL binds mycobacteria strongly and has a role in the uptake of mycobacterium by phagocytes. MBL insufficiency due to polymorphisms in the MBL-2 gene leads to defective opsonization and increases the incidence of recurrent infections in children and adults. [81, 82, 83]
CD14 is known to play a pivotal role in innate immunity by acting as a multifunctional receptor for bacterial cell wall components. CD14 also is involved in TLRs-mediated signaling. [84]
Polymorphisms of the NRAMP-INT4, mannose-binding lectin (MBL codons 52, 54, 57), and CD14-159 genes were explored in a study population of 250 Caucasian Polish individuals. The results suggested a possible role of CD14 and MBL molecules in the host-mycobacteria interactions. An increased serum CD14 count and MBL concentrations were noted in TB patients compared to healthy controls. [81]
SP110
The product of this gene interacts with the interferon system and is an important aspect of the immune response. A study of 27 different polymorphisms in this gene found 3 associated with increased risk for TB; 2 of these polymorphisms were intronic, and the third was a missense mutation in exon 1134.
Cytokine-inducible SRC homology 2 (SH2) domain protein (CISH)
The product of this gene functions to suppress cytokine signaling, an important part of inflammatory cascades. One study found that a single-nucleotide polymorphism upstream from CISH was associated with susceptibility to TB, malaria, and invasive bacterial disease. The same study found that leukocytes of persons with the risk variant for CISH had a decreased response to interleukin 235.
Immunity-related GTPase family M protein (IRGM)
The expression of this gene is induced by interferon, and the product is involved in the control of intracellular mycobacteria. In vitro analyses revealed increased expression of the IRGM gene product with the promoter variant, further underscoring the importance of this gene in the immune response to mycobacterial infection. One study found that homozygosity for a particular polymorphism in the promoter region of IRGM confers protection against TB, but only in persons of European ancestry. [85] Another study by King et al (2011) reported that the CD-related IRGM1 polymorphism is associated with increased susceptibility to TB disease among African Americans. [86]
Interferon-gamma (IFNG)
Interferon-gamma is a cytokine that has an important role in the immune response to intracellular infections, including viral and mycobacterial infections. It activates macrophages to kill the intracellular bacteria through reactive nitrogen and oxygen intermediates and inducing phagosome formation. [87, 88] One polymorphism near a microsatellite in the NF kappa B binding site is associated with increased IFNG gene expression and interferon-gamma production. This polymorphism was related to protection against TB, as seen in a subsequent study. [89]
Interferon gamma receptor-1 (IFNGR1)
The product of IFNGR1 is part of a heterodimeric receptor for interferon-gamma. This has important implications for the response of this part of the immune system in the defense against certain infections.
A region of homozygosity in the region of the IFNGR1 gene has been found in a group of related children in southern Europe who were known to have a predisposition to mycobacterial infection; this predisposition, which had resulted in death in three children and chronic mycobacterial infection in a fourth, was felt to be autosomal recessive. [90] Subsequent gene sequencing showed a nonsense mutation that resulted in a nonfunctional gene product. [91]
TIR domain-containing adaptor protein (TIRAP)
Toll-like receptors (TLRs) recognize a variety of pathogens and initiate intracellular signaling through their Toll/Interleukin-1 receptor (TIR)-domains. Upon translation and activation, the adaptor protein TIRAP (TIR domain-containing adaptor protein), also known as MAL (MYD88 adapter-like), activates transcription factor NF-κB and the rest of the pro-inflammatory genes. A study of 33 polymorphisms in the TIRAP gene found that heterozygosity for a serine-to-leucine substitution was associated with protection against invasive pneumococcal disease, bacteremia, malaria, and TB. [92]
CD209
The product of the CD209 gene is involved in the function of dendritic cells, which are involved in capturing certain microorganisms. An association was found between susceptibility to TB and a polymorphism upstream from the CD209 gene in a multiracial South African population. [93]
HLA and non HLA genes
Human leukocyte antigen (HLA) and non-HLA genes also are reported in association with increased susceptibility to TB and are being studied as potential genetic markers. HLA studies from India have demonstrated an association of HLA-DR2 and HLA-DQ1 with increased susceptibility to pulmonary tuberculosis. [75]
Epidemiology
Occurrence in the United States
With the improvement of living conditions and the introduction of effective treatment (streptomycin) in the late 1940s, the number of patients in the United States with reported TB began to steadily decline (126,000 TB patients in 1944; 84,000 in 1953; 22,000 in 1984; 14,000 in 2004), despite explosive growth in the total population (140 million people in 1946, 185 million in 1960, 226 million in 1980).
In 2022, 8,300 TB cases were reported in the USA, compared with 7,874 cases in 2021. TB incidence increased slightly in 2022 (2.5 cases per 100,000 persons) after an artificially generated substantial decline in 2020. This drop was associated with the COVID-19 pandemic-induced social distancing, masking, and missed or delayed diagnoses. Now, after the social distancing restrictions are lifted, and people are seeking health care increasingly, reported TB cases and TB incidence in the United States are returning to pre-pandemic levels. According to the 2023 CDC report, an estimated 13 million people are living with latent TB in the USA. [94]
In terms of demographics, the majority of reported TB cases occurred among non-US–born persons (71.4%), with the incidence rate being 15.8 times higher among non-US–born persons (12.5 cases per 100,000 persons) than US-born persons (0.8 cases per 100,000 persons). Among non-US–born persons with TB disease in 2021, the most common countries of birth included Mexico, the Philippines, India, Vietnam, and China. Among non-US–born persons, the countries of birth with the highest US incidence rates (cases per 100,000 persons from the country population living in the United States) of TB disease were the Republic of the Marshall Islands, the Republic of the Congo, Mongolia, Bhutan, Myanmar, and Somalia. In 2021, among the persons with TB disease in the United States, 36% identified as non-Hispanic Asian persons. The remainder included Hispanic or Latino persons (30.6%), non-Hispanic, African Americans (18.0%), and non-Hispanic White persons (11.2%). TB incidence rates are higher among adults than among children. Among individuals 15 years and older, the incidence rates increase with age, with adults 65 years or older having the highest TB incidence rate in 2021 (4.0 cases per 100,000 persons). In 2021, males accounted for 61.3% of TB cases in the United States, including 62.6% of cases among US-born persons and 60.7% among non-US–born persons. [95]
Prognosis
With completed therapy, a full resolution generally is expected with few complications in cases of non-MDR- and non-XDR-TB. Among published studies, the recurrence rate in patients involved in direct observed therapy(DOT) treatment ranges from 0-14%. [96] In countries with low TB rates, recurrences usually occur within 12 months of treatment completion and are due to relapse. [97] In countries with higher TB rates, most recurrences after appropriate treatment probably are due to reinfection rather than relapse. [98]
Extrapulmonary involvement, an immunocompromised state, older age, and previous infection/treatment history are considered poor prognostic markers. In a prospective study of 199 patients with TB in Malawi, 12 (6%) died. Risk factors for mortality were reduced baseline TNF-α response to stimulation (with heat-killed Mtb), low body mass index, and elevated respiratory rate at TB diagnosis. [99]
-
Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis.
-
This radiograph shows a patient with typical radiographic findings of tuberculosis.
-
This is a chest radiograph taken after therapy was administered to a patient with tuberculosis.
-
Anteroposterior chest radiograph of a young patient who presented to the emergency department (ED) with cough and malaise. The radiograph shows a classic posterior segment right upper lobe density consistent with active tuberculosis. This woman was admitted to isolation and started empirically on a 4-drug regimen in the ED. Tuberculosis was confirmed on sputum testing. Image courtesy of Remote Medicine (remotemedicine.org).
-
Lateral chest radiograph of a patient with posterior segment right upper lobe density consistent with active tuberculosis. Image courtesy of Remote Medicine (remotemedicine.org).
-
Pulmonary tuberculosis with air-fluid level.
-
Under a high magnification of 15549x, this scanning electron micrograph depicts some of the ultrastructural details seen in the cell wall configuration of a number of Gram-positive Mycobacterium tuberculosis bacteria. As an obligate aerobic organism, M. tuberculosis can only survive in an environment containing oxygen. This bacterium ranges in length between 2-4 microns, with a width between 0.2-0.5 microns. Image courtesy of the Centers for Disease Control and Prevention/Dr. Ray Butler.
-
Numerous acid-fast bacilli (pink) from a bronchial wash are shown on a high-power oil immersion.
-
Necrotizing granuloma due to tuberculosis shown on low-power hematoxylin and eosin stain. There is central caseous necrosis and a multinucleated giant cell in the central left. Mixed inflammation is seen in the background.
-
This chest radiograph shows asymmetry in the first costochondral junctions of a 37-year-old man who presented with cough and fever. Further clarification with computed tomography is needed.
-
Axial noncontrast enhanced computed tomography with pulmonary window shows a cavity with an irregular wall in the right apex of a 37-year-old man who presented with cough and fever (same patient as above).
-
Coronal reconstructed computed tomography image shows the right apical cavity in a 37-year-old man who presented with cough and fever (same patient as above).
-
This posteroanterior chest radiograph shows right upper lobe consolidation with minimal volume loss (elevated horizontal fissure) and a cavity in a 43-year-old man who presented with cough and fever.
-
Axial chest computed tomography without intravenous contrast with pulmonary window setting shows a right apical thick-walled cavity and surrounding lung consolidation in a 43-year-old man who presented with cough and fever (same patient as above).
-
Coronal reconstructed computed tomography image shows the consolidated, partially collapsed right upper lobe with a cavity that is directly connected to a bronchus in a 43-year-old man who presented with cough and fever (same patient as above).
-
The posteroanterior chest radiograph shows a large cavity with surrounding consolidation in the lingular portion of the left upper lobe in a 43-year-old man who presented with cough and hemoptysis. There are also a few nodular opacities in the right mid-lung zone.
-
Axial chest computed tomography without intravenous contrast with pulmonary window setting through the mid-chest shows a large, irregular-walled cavity with nodules and air-fluid level and two smaller cavities in a 43-year-old man who presented with cough and hemoptysis (same patient as above). Small, patchy peripheral opacities are also present in the left lower lobe. In the right mid-lung, nodular opacities are in a tree-in-bud distribution, suggestive of endobronchial spread.
-
Coronal reconstructed computed tomography image shows the lingular cavity with irregular nodules and right mid-lung nodular opacities in a 43-year-old man who presented with cough and hemoptysis (same patient as above).