Practice Essentials
Beta-adrenergic antagonist (ie, beta-blocker) toxicity can produce clinical manifestations including bradycardia, hypotension, arrhythmias, hypothermia, hypoglycemia, and seizures (see the images below). The presentation may range from asymptomatic to shock.
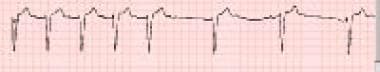 Beta-blocker toxicity. Bradycardia is evident on a rhythm strip from a 48-year-old man who presented to the emergency department after a generalized tonic-clonic seizure. The patient was also hypotensive (82/55 mm Hg). The family reported that he was taking a medication, which proved to be propranolol, for a rapid heart rate. Propranolol is the most common beta-blocker involved in severe beta-blocker poisoning. It is nonselective and has membrane-stabilizing effects that are responsible for CNS depression, seizures, and prolongation of the QRS complex.
Beta-blocker toxicity. Bradycardia is evident on a rhythm strip from a 48-year-old man who presented to the emergency department after a generalized tonic-clonic seizure. The patient was also hypotensive (82/55 mm Hg). The family reported that he was taking a medication, which proved to be propranolol, for a rapid heart rate. Propranolol is the most common beta-blocker involved in severe beta-blocker poisoning. It is nonselective and has membrane-stabilizing effects that are responsible for CNS depression, seizures, and prolongation of the QRS complex.
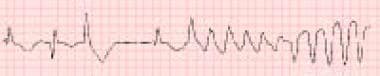 Beta-blocker toxicity. Sotalol is associated with the rhythm shown below in both therapeutic doses and toxic ingestions. Sotalol has been used as a class III antiarrhythmic agent to control dangerous ventricular tachydysrhythmias in some individuals. It causes polymorphic ventricular tachycardia (torsade de pointes) in approximately 4% of patients. Rarely, prolongation of the QT interval has been reported with propranolol.
Beta-blocker toxicity. Sotalol is associated with the rhythm shown below in both therapeutic doses and toxic ingestions. Sotalol has been used as a class III antiarrhythmic agent to control dangerous ventricular tachydysrhythmias in some individuals. It causes polymorphic ventricular tachycardia (torsade de pointes) in approximately 4% of patients. Rarely, prolongation of the QT interval has been reported with propranolol.
Beta-blockers have been in use for nearly 50 years. In addition to their traditional role in treating hypertension and other cardiovascular disorders, beta-blockers are also used for additional purposes such as migraine headaches, hyperthyroidism, glaucoma, anxiety, and various other disorders. As a result of their expanded use, the incidence of overdose with these agents has also increased.
Beta-blocker toxicity in children usually results from exposure to an adult's unattended medications. Beta-blocker toxicity in adults usually results from a suicide attempt or an accidental overdose of a routine medication.
Pathophysiology
Understanding the direct and indirect effects of beta-receptor blockade is crucial to rapid identification and appropriate treatment of beta-blocker toxicity. Beta-blockers act as competitive inhibitors of catecholamines, exerting their effects at both central and peripheral receptors. Blockade of beta-receptors results in decreased production of intracellular cyclic adenosine monophosphate (cAMP) with a resultant blunting of multiple metabolic and cardiovascular effects of circulating catecholamines.
Beta1-receptor blockade reduces heart rate, blood pressure, myocardial contractility, and myocardial oxygen consumption. Beta2-receptor blockade inhibits relaxation of smooth muscle in blood vessels, bronchi, the gastrointestinal system, and the genitourinary tract. In addition, beta-adrenergic receptor antagonism inhibits both glycogenolysis and gluconeogenesis, which may result in hypoglycemia.
Other than the direct effects of the beta-adrenoreceptor blockade, toxicity may result from other mechanisms, including sodium and calcium channel blockade, centrally mediated cardiac depression, and alteration of cardiac myocyte energy metabolism. [1]
Numerous beta-blockers are available; these agents comprise a heterogeneous drug family with varying toxicologically relevant characteristics. An understanding of these different characteristics is helpful for understanding the clinical presentations with particular agents and for guiding therapy.
Nonselective activity
Propranolol was the first beta-blocker to enter widespread use; much of the clinical and overdose experience that exists with beta-blockers was provided by case reports and clinical studies of this drug. Propranolol is a nonselective beta-blocker, demonstrating equal affinity for both beta1- and beta2-receptors. Other nonselective beta-blockers include nadolol, timolol, and pindolol. Nonselective beta-blockers exert a wider variety of extracardiac manifestations.
Intrinsic sympathomimetic activity
Some beta-blockers, such as pindolol and acebutolol, also have beta-agonist properties. Although their agonist property is weaker than that of catecholamines, they are capable of stimulating beta-receptors, especially when catecholamine levels are low. Of note, acebutolol has been reported to be particularly lethal in overdose.
Membrane-stabilizing activity
Beta-blockers, such as propranolol, labetalol, and pindolol, can have membrane-stabilizing activity (MSA; eg, the quinidine-like effects of the class IA antidysrhythmic effects). MSA blocks myocyte sodium channels. This property, usually not evident at therapeutic doses, may significantly contribute to toxicity by prolonging QRS duration and impairing cardiac conduction. Seizures are more commonly observed in drugs with MSA. Beta-blockers with MSA are associated with the largest proportion of fatalities.
Lipid solubility
Lipid solubility is higher in agents such as propranolol and carvedilol, but lower in agents such as atenolol and nadolol. It may influence the degree of central nervous system (CNS) effects and utility of hemodialysis or hemoperfusion. [2]
High lipid solubility leads to a larger volume of distribution and better CNS penetration. Lipophilic beta-blockers are primarily metabolized by the liver. Propranolol is among these, and its active metabolite (4-OH propranolol) prolongs its biological activity. Conversely, hydrophilic beta-blockers have a small volume of distribution and are eliminated essentially unchanged by the kidneys; this property allows hydrophilic beta-blockers to be removed by hemodialysis.
QT-interval prolongation
The electrophysiologic effects of sotalol deserve special consideration. Unlike other beta-blockers, sotalol has antidysrhythmic properties consistent with the type III antidysrhythmic agents. Class III agents prolong the action potential duration and the effective refractory period of AV and atrioventricular myocytes, which can lengthen the QT-interval duration and result in polymorphic ventricular tachycardia (ie, torsade de pointes). Ventricular dysrhythmias associated with sotalol toxicity can occur up to 48 hours postingestion.
Epidemiology
Propranolol is the most toxic beta-blocker and the most frequently used in suicide attempts worldwide. The 2021 Annual Report of the American Association of Poison Control Centers' (AAPCC) National Poison Data System reported 10,832 single exposures to beta-blockers. Of the reported exposures, 2452 were in children younger than 6 years, and 6894 were in adults 20 years of age and older. Approximately 80% of exposures were unintentional. [3]
In a single-site review from Iran of adults hospitalized due to poisoning, 255 (5.1%) of the 5086 cases involved beta-blocker toxicity. The majority of patients were women (80.6%), and almost all (95.3%) of the exposures were intentional. Propranolol was present in 84.4% of cases and 62% of patients had exposure to multiple beta-blockers. [4]
Prognosis
Prognosis is largely dependent on the initial response to therapy (6-12 h postingestion), as drug levels are likely to have peaked at this time. In addition, beta-blockers that are lipid soluble and have marked antidysrhythmic (ie, quinidine-like) effects are more lethal (eg, propranolol, sotalol, oxprenolol).
Underlying cardiac or pulmonary disease places the patient at increased risk for poor outcome.
The outcome is significantly worse when these agents are co-ingested with psychotropic or cardioactive drugs. This is true even if the amount of beta-blocker ingested is relatively small. The co-ingestants that most markedly worsen prognosis include calcium channel blockers, cyclic antidepressants, and neuroleptics. These co-ingestions are the most important factor associated with the development of cardiovascular morbidity and mortality.
Although beta-blockers were the seventh leading cause of fatal exposure in the 2021 AAPCC report, the overall fatality rate was low: 0.42%. The following outcomes in 4268 cases of beta-blocker exposure treated in a healthcare facility were reported [3] :
-
None - 3508
-
Minor - 731
-
Moderate - 1094
-
Major - 144
-
Deaths - 18
-
Beta-blocker toxicity. Bradycardia is evident on a rhythm strip from a 48-year-old man who presented to the emergency department after a generalized tonic-clonic seizure. The patient was also hypotensive (82/55 mm Hg). The family reported that he was taking a medication, which proved to be propranolol, for a rapid heart rate. Propranolol is the most common beta-blocker involved in severe beta-blocker poisoning. It is nonselective and has membrane-stabilizing effects that are responsible for CNS depression, seizures, and prolongation of the QRS complex.
-
Beta-blocker toxicity. Sotalol is associated with the rhythm shown below in both therapeutic doses and toxic ingestions. Sotalol has been used as a class III antiarrhythmic agent to control dangerous ventricular tachydysrhythmias in some individuals. It causes polymorphic ventricular tachycardia (torsade de pointes) in approximately 4% of patients. Rarely, prolongation of the QT interval has been reported with propranolol.









