Practice Essentials
Sepsis is defined as life-threatening organ dysfunction due to dysregulated host response to infection, and organ dysfunction is defined as an acute change in total Sequential Organ Failure Assessment (SOFA) score of 2 points or greater secondary to the infection cause. [1] Septic shock occurs in a subset of patients with sepsis and comprises of an underlying circulatory and cellular/metabolic abnormality that is associated with increased mortality. Septic shock is defined by persisting hypotension requiring vasopressors to maintain a mean arterial pressure of 65 mm Hg or higher and a serum lactate level greater than 2 mmol/L (18 mg/dL) despite adequate volume resuscitation. [1] This new 2016 definition, also called Sepsis-3, eliminates the requirement for the presence of systemic inflammatory response syndrome (SIRS) to define sepsis, and it removed the severe sepsis definition. What was previously called severe sepsis is now the new definition of sepsis.
Signs and symptoms
Detrimental host responses to infection occupy a continuum that ranges from sepsis to septic shock and multiple organ dysfunction syndrome (MODS). The specific clinical features depend on where the patient falls on that continuum.
Signs and symptoms of sepsis are often nonspecific and include the following:
-
Fever (usually >101°F [38°C]), chills, or rigors
-
Confusion
-
Anxiety
-
Difficulty breathing
-
Fatigue, malaise
-
Nausea and vomiting
In sepsis, symptoms may include decreased urine output and cyanosis (blueish discoloration of the lips and/or digits).
Alternatively, typical symptoms of systemic inflammation may be absent in sepsis, especially in elderly individuals.
It is important to identify any potential source of infection. Localizing signs and symptoms referable to organ systems may provide useful clues to the etiology of sepsis and are as follows:
-
Head and neck infections – Severe headache, neck stiffness, altered mental status, earache, sore throat, sinus pain/tenderness, cervical/submandibular lymphadenopathy
-
Chest and pulmonary infections – Cough (especially if productive), pleuritic chest pain, dyspnea, dullness on percussion, bronchial breath sounds, localized rales, any evidence of consolidation
-
Cardiac infections – Any new murmur, especially in patients with a history of injection or IV drug use
-
Abdominal and gastrointestinal (GI) infections – Diarrhea, abdominal pain, abdominal distention, guarding or rebound tenderness, rectal tenderness or swelling
-
Pelvic and genitourinary (GU) infections – Pelvic or flank pain, adnexal tenderness or masses, vaginal or urethral discharge, dysuria, frequency, urgency
-
Bone and soft-tissue infections – Localized limb pain or tenderness, focal erythema, edema, swollen joint, crepitus in necrotizing infections, joint effusions
-
Skin infections – Petechiae, purpura, erythema, ulceration, bullous formation, fluctuance
See Clinical Presentation for more detail.
Diagnosis
Patients with sepsis may present in a myriad of ways, and a high index of clinical suspicion is necessary to identify subtle presentations. The hallmarks of sepsis and septic shock are changes that occur at the microvascular and cellular level and may not be clearly manifested in the vital signs or clinical examination. This process includes diffuse activation of inflammatory and coagulation cascades, vasodilation and vascular maldistribution, capillary endothelial leakage, and dysfunctional utilization of oxygen and nutrients at the cellular level.
Cardiac monitoring, noninvasive blood pressure monitoring, and pulse oximetry are indicated in patients with septic shock.
Laboratory tests
The following are investigative studies to detect a clinically suspected focal infection, the presence of a clinically occult focal infection, and complications of sepsis and septic shock:
-
Complete blood count with differential
-
Coagulation studies (eg, prothrombin time [PT], activated partial thromboplastin time [aPTT], fibrinogen levels)
-
Blood chemistry (eg, sodium, chloride, magnesium, calcium, phosphate, glucose, lactate)
-
Renal and hepatic function tests (eg, creatinine, blood urea nitrogen, bilirubin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, albumin, lipase)
-
Blood cultures
-
Urinalysis and urine cultures
-
Gram stain and culture of secretions and tissue
Imaging studies
The following radiologic studies, as indicated, may be used to evaluate patients with suspected sepsis and septic shock:
-
Chest, abdominal, or extremity radiography
-
Abdominal ultrasonography
-
Computed tomography of the abdomen or head
Lumbar puncture
A lumbar puncture/spinal fluid test is indicated in the following circumstances:
-
Clinical evidence or suspicion of meningitis
-
Clinical evidence or suspicion of encephalitis
See Workup for more detail.
Management
Patients with sepsis and septic shock require admission to the hospital. Initial treatment includes support of respiratory and circulatory function, supplemental oxygen, mechanical ventilation, and volume infusion.
Treatment of patients with septic shock has the following major goals:
-
Start adequate antibiotics (proper spectrum and dose) as early as possible
-
Resuscitate the patient from septic shock by using supportive measures to correct hypoxia, hypotension, and impaired tissue oxygenation (hypoperfusion)
-
Identify the source of infection and treat with antimicrobial therapy, surgery, or both (source control)
-
Maintain adequate organ system function, guided by cardiovascular monitoring, and interrupt the progression of MODS
Management principles for septic shock include the following:
-
Early recognition
-
Early and adequate antibiotic therapy
-
Source control
-
Early hemodynamic resuscitation and continued support
-
Proper ventilator management with low tidal volume in patients with acute respiratory distress syndrome (ARDS)
Pharmacotherapy
The following medications may be used in the management of septic shock:
-
Alpha-/beta-adrenergic agonists (eg, norepinephrine, dopamine, dobutamine, epinephrine, vasopressin, phenylephrine)
-
Synthetic human angiotensin II
-
Isotonic crystalloids (eg, balanced salt solutions, lactated Ringer solution)
-
Volume expanders (eg, albumin)
-
Antibiotics (eg, cefotaxime, ticarcillin-clavulanate, piperacillin-tazobactam, imipenem-cilastatin, meropenem, clindamycin, metronidazole, ceftriaxone, ciprofloxacin, cefepime, levofloxacin, vancomycin)
-
Corticosteroids (eg, hydrocortisone, dexamethasone)
Surgery
Patients with focal infections should be sent for definitive surgical treatment after initial resuscitation and administration of antibiotics. [2] However, although urgent management is indicated for hemodynamically stable patients without evidence of acute organ failure, delay of invasive procedures for as long as 24 hours may be possible if the patient receives very close clinical monitoring and appropriate antimicrobial therapy. [2]
Certain conditions will not respond to standard treatment for septic shock until the source of infection is surgically removed (eg, intra-abdominal sepsis [perforation, abscesses], empyema, mediastinitis, cholangitis, pancreatic abscesses, pyelonephritis or renal abscess from ureteric obstruction, infective endocarditis, septic arthritis, infected prosthetic devices, deep cutaneous or perirectal abscess, and necrotizing fasciitis).
When possible, percutaneous drainage of abscesses and other well-localized fluid collections is preferred to surgical drainage. [2] However, any deep abscess or suspected necrotizing fasciitis should undergo drainage in the surgical suite.
See Treatment and Medication for more detail.
Background
Over many years, the terms sepsis and septicemia have referred to several ill-defined clinical conditions present in a patient with bacteremia. Definitions have not changed greatly since 1914, when Schottmueller wrote, “Septicemia is a state of microbial invasion from a portal of entry into the blood stream which causes sign of illness.”
In practice, these two terms have often been used interchangeably; however, only about half of patients with signs and symptoms of sepsis have positive results on blood culture. [3, 4, 5] Furthermore, not all patients with bacteremia have signs of sepsis. It follows, therefore, that sepsis and septicemia are not in fact identical.
In the past few decades, the discovery of endogenous mediators of the host response has led to the recognition that the clinical syndrome of sepsis is the result of excessive activation of host defense mechanisms rather than the direct effect of microorganisms. Sepsis and its sequelae represent a continuum of clinical and pathophysiologic severity.
Serious bacterial infections at any site in the body (see the image below), with or without bacteremia, are usually associated with important changes in the function of every organ system in the body. These changes are mediated mostly by elements of the host immune system against infection. Shock is deemed present when volume replacement fails to increase blood pressure to acceptable levels and when associated clinical evidence indicates inadequate perfusion of major organ systems, with progressive failure of organ system functions. Although hyperlactecemia is commonly seen in sepsis, its relationship to hypoperfusion is questionable and is more often due to the acute inflammatory state, impaired lactate clearance, and nonoxidative phosphorylation lactate production.
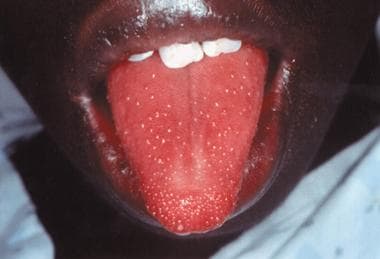 Strawberry tongue in a child with staphylococcal toxic shock syndrome. Reproduced with permission from Drage, LE. Life-threatening rashes: dermatologic signs of four infectious diseases. Mayo Clin Proc. 1999;74:68-72.
Strawberry tongue in a child with staphylococcal toxic shock syndrome. Reproduced with permission from Drage, LE. Life-threatening rashes: dermatologic signs of four infectious diseases. Mayo Clin Proc. 1999;74:68-72.
Multiple organ dysfunctions, the extreme end of the continuum, are incremental degrees of physiologic derangements in individual organs (ie, processes rather than events). Alteration in organ function can vary widely, ranging from a mild degree of organ dysfunction to frank organ failure. (See Multiple Organ Failure of Sepsis, Systemic Inflammatory Response Syndrome (SIRS), Toxic Shock Syndrome, and Septic Thrombophlebitis .)
This article does not cover sepsis of the neonate or infant. Special consideration must be given to neonates, infants, and small children with regard to fluid resuscitation, appropriate antibiotic coverage, intravenous (IV) access, and vasopressor therapy. (See Neonatal Sepsis, Pediatric Sepsis, Treatment of Sepsis and Septic Shock in Children, Shock in Pediatrics, and Shock and Hypotension in the Newborn.)
Shock Classification, Terminology, and Staging
Shock is identified in most patients by hypotension and inadequate organ perfusion, which may be caused by either low cardiac output or low systemic vascular resistance. Circulatory shock can be subdivided into four distinct classes on the basis of underlying mechanism and characteristic hemodynamics, as follows:
-
Hypovolemic shock
-
Obstructive shock
-
Distributive shock
-
Cardiogenic shock
These classes of shock should be considered and systematically differentiated before a definitive diagnosis of septic shock is established.
Hypovolemic shock results from the loss of blood volume caused by such conditions as gastrointestinal (GI) bleeding, extravasation of plasma, major surgery, trauma, and severe burns. Patients suffering from hypovolemic shock demonstrate tachycardia, cool clammy extremities, hypotension, dry skin and mucous membranes, and poor turgor.
Obstructive shock results from an intrinsic or extrinsic obstruction of circulation. Pulmonary embolism and pericardial tamponade both result in obstructive shock.
Distributive shock is caused by excessive vasodilation and impaired distribution of blood flow (eg, direct arteriovenous shunting), and it is characterized by decreased resistance or increased venous capacity from the vasomotor dysfunction. Patients with this type of shock have high cardiac output, hypotension, a large pulse pressure, a low diastolic pressure, and warm extremities with good capillary refill. These findings on physical examination strongly suggest a working diagnosis of septic shock.
Cardiogenic shock is characterized by primary myocardial dysfunction, which renders the heart unable to maintain adequate cardiac output. Affected patients demonstrate clinical signs of low cardiac output while showing evidence of adequate intravascular volume. The patients have cool clammy extremities, poor capillary refill, tachycardia, a narrow pulse pressure, and low urine output.
Definitions of key terms
The basis of sepsis is the presence of infection associated with a systemic inflammatory response that results in physiologic alterations at the capillary endothelial level. The difficulty in diagnosis comes in knowing when a localized infection has become systemic and requires more aggressive hemodynamic support. No criterion standard exists for the diagnosis of endothelial dysfunction, and patients with sepsis may not initially present with frank hypotension and overt shock.
Clinicians often use the terms sepsis, severe sepsis, and septic shock without following commonly understood definitions. In 1991, the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) convened a consensus conference to establish definitions of these and related terms. [6, 7] The most recent consensus was published in 2016. [1]
Systemic inflammatory response syndrome
The term systemic inflammatory response syndrome (SIRS) was developed in an attempt to describe the clinical manifestations that result from the systemic response to infection (fever or hypothermia, tachycardia, tachypnea, and hyperleukocytosis or leukopenia). Criteria for SIRS are considered to be met if at least 2 of the following 4 clinical findings are present:
-
Temperature higher than 38°C (100.4°F) or lower than 36°C (96.8°F)
-
Heart rate (HR) higher than 90 beats/min
-
Respiratory rate (RR) higher than 20 breaths/min or arterial carbon dioxide tension (PaCO2) lower than 32 mm Hg
-
White blood cell (WBC) count higher than 12,000/µL or lower than 4000/µL or with 10% immature (band) forms
Note that a patient can have a severe infection without meeting SIRS criteria; conversely, SIRS criteria may be present in the setting of many other illnesses not caused by an infectious process (see the image below).
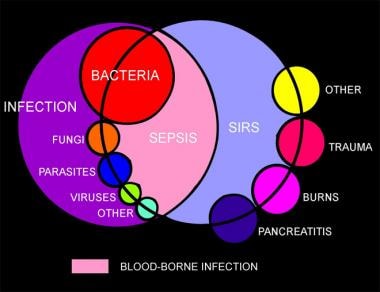 Venn diagram showing the overlap of infection, bacteremia, sepsis, systemic inflammatory response syndrome (SIRS), and multiorgan dysfunction.
Venn diagram showing the overlap of infection, bacteremia, sepsis, systemic inflammatory response syndrome (SIRS), and multiorgan dysfunction.
In 2001, as a follow-up to the original ACCP/SCCM conference, an International Sepsis Definitions Conference was convened, with representation not only from the ACCP and the SCCM but also from the European Society of Intensive Care Medicine (ESICM), the American Thoracic Society (ATS), and the Surgical Infection Society (SIS). The following definitions of sepsis syndromes were published to clarify the terminology used to describe the spectrum of disease that results from severe infection. [8] In 2016, a new consensus termed Sepsis-3 removed the concept of SIRS from the sepsis definition and replaced it with the SOFA score. [1] This change increased specificity but decreased sensitivity. However, the authors stated that the SIRS criteria should continue to aid in the general diagnosis of infection.
Sepsis
Sepsis is defined as life-threatening organ dysfunction due to dysregulated host response to infection, and organ dysfunction is defined as an acute change in total SOFA score of 2 points or greater secondary to the infection cause (see Table 1 below). [1]
Table 1. Sepsis-Related SOFA Score (adapted from Singer et al) (Open Table in a new window)
System |
0 Points |
1 Point |
2 Points |
3 Points |
4 Points |
Respiration PaO2a/FiO2b |
≥400 mm Hg |
< 400 mm Hg |
< 300 mm Hg |
< 200 mm Hg (with respiratory support) |
< 100 mm Hg (with respiratory support) |
Coagulation Platelet count |
≥150 x 103/µL |
< 150 x 103/µL |
< 100 x 103/µL |
< 50 x 103/µL |
< 20 x 103/µL |
Liver Bilirubin level |
< 1.2 mg/dL |
1.2-1.9 mg/dL |
2-5.9 mg/dL |
6-11.9 mg/dL |
>12 mg/dL |
Cardiovascular |
MAPc ≥70 mm Hg |
MAP >70 mm Hg |
Dopamine < 5 or dobutamine (any dose)e |
Dopamine 5.1-15 or epinephrine ≤0.1 or norepinephrine ≤0.1e |
Dopamine >15 or epinephrine >0.1 or norepinephrine >0.1e |
Central nervous system GCSd score |
15 |
13-14 |
10-12 |
6-9 |
< 6 |
Renal Creatinine Urine output |
< 1.2 mg/dL |
1.2-1.9 mg/dL |
2-3.4 mg/dL |
3.5-4.9 mg/dL < 500 mL/day |
>5 mg/dL < 200 mL/day |
aPaO2=Partial pressure of oxygen. bFiO2=Fraction of inspired oxygen. cMAP=Mean arterial pressure. dGCS=Glasgow Coma Scale (range, 3-15, with higher indicating better function). eCatecholamine doses administered as µg/kg/min for ≥1 hour. |
|||||
Sepsis-induced organ dysfunction is defined by an acute change in total SOFA score of 2 points or greater secondary to the infection cause. [1] For screening purposes, a shorter version of the SOFA score, termed quick SOFA (qSOFA), demonstrated to have reasonable accuracy in the settings outside the ICU. [1] The qSOFA is defined by two or more of a total of the following three components: altered mental status, respiratory rate of 22 or higher, and systolic blood pressure of 100 mm Hg or less. While the qSOFA is not as robust as the total SOFA score, there is no requirement for laboratory tests and easier reassessment make the qSOFA a potential tool for screening a possible infection as a source of a new sepsis episode in settings with lower resources than standard ICUs. However, the qSOFA still needs prospective validation in future cohort studies.
Septic shock
Septic shock occurs in a subset of patients with sepsis and comprises of an underlying circulatory and cellular/metabolic abnormality that is associated with increased mortality. Septic shock is defined by persistent hypotension requiring vasopressors to maintain mean a arterial pressure of 65 mm Hg or higher and a serum lactate level greater than 2 mmol/L (18 mg/dL) despite adequate volume resuscitation. [1]
Bacteremia
Bacteremia is defined as the presence of viable bacteria within the liquid component of blood (blood infection). It may be primary (without an identifiable focus of infection) or, more often, secondary (with an intravascular or extravascular focus of infection). Although sepsis is associated with bacterial infection, bacteremia is not a necessary ingredient in the activation of the inflammatory response that results in sepsis. In fact, septic shock is associated with culture-positive bacteremia in only 30-50% of cases. [3, 4, 5]
Multiple organ dysfunction syndrome
Multiple organ dysfunction syndrome (MODS) is defined as the presence of altered organ function in a patient who is acutely ill and in whom homeostasis cannot be maintained without intervention. MODS may eventually lead to multiple organ failure syndrome (MOFS) and death. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common manifestations of MODS or MOFS. However, other conditions besides sepsis can cause MODS, including trauma, burns, and severe hemorrhagic shock.
Acute lung injury and acute respiratory distress syndrome
In 1994, the American-European Consensus Conference on ARDS agreed on standard definitions of ALI and ARDS. [9] However, these definitions were subsequently replaced by the following consensus, referred to as the Berlin Definition of ARDS, which essentially does away with the classification of ALI in favor of classifying ARDS as mild, moderate, or severe [10] :
-
Mild ARDS – An oxygenation abnormality with a PaO2/FIO2 ratio of 200-300 and a positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) of 5 cm water or higher
-
Moderate ARDS – A PaO2/FIO2 ratio of 100-200 and a PEEP of 5 cm water or higher
-
Severe ARDS – A PaO2/FIO2 ratio of 100 or less and a PEEP of 5 cm water or higher
-
Bilateral opacities on chest radiographs that are not fully explained by effusions, lobar/lung collapse, or nodules
-
Edema not of cardiac origin or caused by fluid overload – In the absence of risk factors for ARDS, this requires objective assessment (eg, via echocardiography)
-
Occurrence within 1 week of a known clinical insult or worsening respiratory symptoms
MODS staging
Two well-defined forms of MODS exist. In either, the development of ALI or ARDS is of key importance to the natural history, though ARDS is the earliest manifestation in all cases.
In the more common form of MODS, the lungs are the predominant, and often the only, organ system affected until very late in the disease. Patients with this form of MODS most often present with a primary pulmonary disorder (eg, pneumonia, aspiration, lung contusion, near-drowning, chronic obstructive pulmonary disease [COPD] exacerbation, hemorrhage, or pulmonary embolism [PE]).
Progression of lung disease occurs to meet the ARDS criteria. Pulmonary dysfunction may be accompanied by encephalopathy or mild coagulopathy and persists for 2-3 weeks. At this time, the patient either begins to recover or progresses to develop fulminant dysfunction in other organ systems. Patients who develop another major organ dysfunction often do not survive.
In the second, less common, form of MODS, the presentation is quite different. Patients affected by this form often have an inciting source of sepsis in organs other than the lung; the most common sources are intra-abdominal sepsis, extensive blood loss, pancreatitis, and vascular catastrophes.
Not only does ALI or ARDS develop early, but dysfunction also develops in other organ systems, including the hepatic, hematologic, cardiovascular, and renal systems and central nervous system (CNS). Patients remain in a pattern of compensated dysfunction for several weeks, then either recover or deteriorate further.
Criteria for mild and severe organ dysfunction have been established by the 2012 Surviving Sepsis Guidelines (see Table 2, below). Of note, even though this is the last update of the Surviving Sepsis Campaign, they still separate sepsis and severe sepsis, which was more recently modified by the Sepsis-3 consensus in 2016. [1]
Table 2. Surviving Sepsis Guidelines Criteria for Organ Dysfunction (Open Table in a new window)
Organ System |
Sepsis Criteria |
Severe Sepsis Criteria |
Pulmonary |
Arterial hypoxemia: PaO2/FIO2< 300 |
Arterial hypoxemia: PaO2/FIO2< 250 in absence of pneumonia and < 200 in presence of pneumonia |
Hepatic |
Hyperbilirubinemia: Plasma total bilirubin >4 mg/dL or 70 µmol/L |
Hyperbilirubinemia: Plasma total bilirubin >2 mg/dL or 34.2 µmol/L |
Renal |
Creatinine increase >0.5 mg/dL or 44.2 µmol/L Acute oliguria: Urine output < 0.5 mL/kg/hr for ≥2 hr despite adequate fluid resuscitation |
Creatinine >2 mg/dL or 176.8 µmol/L Acute oliguria: Urine output < 0.5mL/kg/hr for ≥2 hr despite adequate fluid resuscitation |
Gastrointestinal |
Ileus: Absent bowel sounds |
|
Hematologic |
INR >1.5, aPTT >60 s, or platelets < 100,000/µL |
INR >1.5 or platelets < 100,000/µL |
Cardiovascular |
Hyperlactatemia >1 mmol/L; decreased capillary refill or mottling Hemodynamic status: SBP < 90 mm Hg, MAP < 70 mm Hg, or SBP decrease >40 mm Hg |
Hyperlactatemia: Above upper limits of laboratory normal Hemodynamic status: SBP < 90 mm Hg, MAP < 70 mm Hg, or SBP decrease >40 mm Hg |
Central nervous system |
Confusion, lethargy, coma |
|
|
|
|
aPTT = activated partial thromboplastin time; FIO2 = fraction of inspired oxygen; INR = international normalized ratio; MAP = mean arterial pressure; PaO2 = partial pressure of oxygen; PEEP = positive end-expiratory pressure; PT = prothrombin time; SBP = systolic blood pressure. Source: Dellinger RP, Levy MM, Rhodes A, et al, for the Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013 Feb;41(2):580-637. [11] |
||
Pathophysiology
The pathophysiology of septic shock is not precisely understood but is considered to involve a complex interaction between the pathogen and the host’s immune system (see the image below). The normal physiologic response to localized infection includes activation of host defense mechanisms that result in the influx of activated neutrophils and monocytes, release of inflammatory mediators, local vasodilation, increased endothelial permeability, and activation of coagulation pathways.
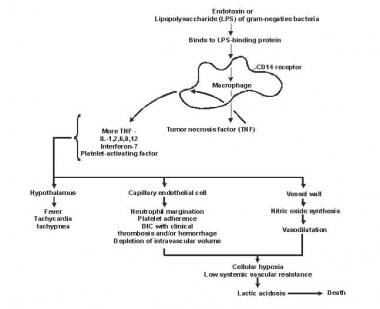 Diagram depicting the pathogenesis of sepsis and multiorgan failure. DIC = disseminated intravascular coagulation; IL = interleukin.
Diagram depicting the pathogenesis of sepsis and multiorgan failure. DIC = disseminated intravascular coagulation; IL = interleukin.
These response mechanisms occur during septic shock, but on a systemic scale, leading to diffuse endothelial disruption, vascular permeability, vasodilation, and thrombosis of end-organ capillaries. Endothelial damage itself can further activate inflammatory and coagulation cascades, creating, in effect, a positive feedback loop and leading to further endothelial and end-organ damage.
Mediator-induced cellular injury
The evidence that sepsis results from an exaggerated systemic inflammatory response induced by infecting organisms is compelling. Inflammatory mediators are the key players in the pathogenesis of sepsis (see Table 3 below).
Table 3. Mediators of Sepsis (Open Table in a new window)
Type |
Mediator |
Activity |
|
Cellular mediators |
LPS |
Activation of macrophages, neutrophils, platelets, and endothelium releases various cytokines and other mediators |
|
Lipoteichoic acid |
|||
Peptidoglycan |
|||
Superantigens |
|||
Endotoxin |
|||
Humoral mediators |
Cytokines |
Activate inflammatory pathways |
|
|
Potent proinflammatory effect |
||
|
Acts as pyrogen, stimulates B- and T-cell proliferation |
||
|
Neutrophil chemotactic factor, activation and degranulation of neutrophils |
||
|
Inhibits cytokine production, induces immunosuppression |
||
|
Activates macrophages and T cells |
||
|
Promotes neutrophil and macrophage, platelet activation |
||
Complement |
Promotes neutrophil and macrophage, platelet activation and chemotaxis, other proinflammatory effects |
||
Nitric oxide |
Involved in hemodynamic alterations of septic shock; cytotoxic, augments vascular permeability, contributes to shock |
|
|
Lipid mediators |
Enhance vascular permeability and contribute to lung injury |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Arachidonic acid metabolites |
Augment vascular permeability |
|
|
Adhesion molecules |
Enhance neutrophil-endothelial cell interaction, regulate leukocyte migration and adhesion, and play a role in pathogenesis of sepsis; increased levels of VAP-1 activity and anchor protein SDC-1 content have been found in critically ill patients with septic shock [12] |
|
|
|
|
|
|
|
|
|
|
|
Late mediator of endotoxin-induced lethality and tissue repair |
|
|
G-CSF = granulocyte colony-stimulating factor; IL = interleukin; LPS = lipopolysaccharide; MIF = macrophage inhibitory factor; PAF = platelet-activating factor; SDC-1 = syndecan-1; TNF = tumor necrosis factor; VAP-1 = vascular adhesion protein–1. Source: Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009 Jan;37(1):291-304. [13] |
|
||
|
|||
Immunologic abnormalities
The following three families of pattern recognition receptors are involved in the initiation of the sepsis response:
-
Toll-like receptors (TLRs)
-
Nucleotide-oligomerization domain leucine-rich repeat proteins
-
Cytoplasmic caspase activation and recruiting domain helicases
These receptors trigger the innate immune response and modulate the adaptive immune response to infection. [13]
An initial step in the activation of innate immunity is the de novo synthesis of small polypeptides (cytokines) that induce protean manifestations on most cell types, from immune effector cells to vascular smooth muscle and parenchymal cells. Several cytokines are induced, including tumor necrosis factor (TNF) and interleukins (ILs), especially IL-1. These factors help keep infections localized; however, once the infection progresses, the effects can also be detrimental.
Circulating levels of IL-6 correlate have a strong correlation with outcome. High levels of IL-6 are associated with mortality, but the role of this cytokine in pathogenesis is not clear. IL-8 is an important regulator of neutrophil function, synthesized and released in significant amounts during sepsis. IL-8 contributes to the lung injury and dysfunction of other organs.
Chemokines (eg, monocyte chemoattractant protein [MCP]-1) orchestrate the migration of leukocytes during endotoxemia and sepsis. Other cytokines thought to play a role in sepsis include the following:
-
IL-10
-
Interferon gamma
-
IL-12
-
Macrophage migration inhibition factor (MIF or MMIF)
-
Granulocyte colony-stimulating factor (G-CSF)
-
Granulocyte macrophage colony-stimulating factor (GM-CSF)
In addition, cytokines activate the coagulation pathway, resulting in capillary microthrombi and end-organ ischemia. [14, 15, 16] (See Abnormalities of coagulation and fibrinolysis, below.)
Gram-positive and gram-negative bacteria induce a variety of proinflammatory mediators, including the cytokines mentioned above, which play a pivotal role in initiating sepsis and shock. Various bacterial cell-wall components are known to release the cytokines, including lipopolysaccharide (LPS; gram-negative bacteria), peptidoglycan (gram-positive and gram-negative bacteria), and lipoteichoic acid (gram-positive bacteria).
Several of the harmful effects of bacteria are mediated by proinflammatory cytokines induced in host cells (macrophages/monocytes and neutrophils) by the bacterial cell-wall component. The most toxic component of gram-negative bacteria is the lipid A moiety of LPS, which leads to cytokine induction via lipoteichoic acid. Additionally, gram-positive bacteria may secrete superantigen cytotoxins that bind directly to the major histocompatibility complex (MHC) molecules and T-cell receptors, leading to massive cytokine production.
The complement system is activated and contributes to the clearance of the infecting microorganisms but probably also enhances the tissue damage. The contact systems become activated; consequently, bradykinin is generated.
Hypotension, the cardinal manifestation of sepsis, occurs via induction of nitric oxide (NO). NO plays a major role in the hemodynamic alterations of septic shock, which is a hyperdynamic form of shock.
In a study that evaluated the role of active nitrogen molecules in the progression of septic shock, investigators found not only that patients with sepsis and septic shock had elevated mean levels of nitrite (NO2)/nitrate (NO3) (sepsis, 78.92 µmol/L; septic shock, 97.20 µmol/L) as well as TNF-α (sepsis, 213.50 pg/mL; septic shock, 227.38 pg/mL) but also that levels of these 3 mediators increased with the severity of the sepsis. [17]
Another factor that contributes to the poor cellular oxygen utilization and tissue organ dysfunction during sepsis is mitochondrial dysfunction. [18] This is associated with excessive generation of peroxynitrates and reactive oxygen species (ROS) in combination with glutathione depletion.
A dual role exists for neutrophils: They are necessary for defense against microorganisms, but they may also become toxic inflammatory mediators, thereby contributing to tissue damage and organ dysfunction. Lipid mediators—eicosanoids, platelet-activating factor (PAF), and phospholipase A2—are generated during sepsis, but their contributions to the sepsis syndrome remain to be established.
Neutrophils are constitutively proapoptotic, a capacity that is essential for the resolution of inflammation and cell turnover. Poor apoptosis is associated with poor cell clearance and a proinflammatory state.
There is a growing body of evidence regarding sepsis-induced immunosuppression, which may culminate in a worse prognosis and a greater predisposition to other nosocomial infections. [19] In addition, there is evidence that patients with sepsis who have previously been infected with cytomegalovirus may have worse outcomes than those who have not. [20] That cytomegalovirus infection can also cause immunomodulation may be another factor contributing to sepsis-induced immunosuppression.
Abnormalities of coagulation and fibrinolysis
An imbalance of homeostatic mechanisms leads to disseminated intravascular coagulopathy (DIC) and microvascular thrombosis, causing organ dysfunction and death. [21] Inflammatory mediators instigate direct injury to the vascular endothelium; the endothelial cells release tissue factor (TF), triggering the extrinsic coagulation cascade and accelerating thrombin production. Plasma levels of endothelial activation biomarkers are higher in patients with sepsis-induced hypotension than in patients with hypotension from other causes. [22]
The coagulation factors are activated as a result of endothelial damage. The process is initiated through binding of factor XII to the subendothelial surface, which activates factor XII. Subsequently, factor XI and, eventually, factor X are activated by a complex of factor IX, factor VIII, calcium, and phospholipid. The final product of the coagulation pathway is the production of thrombin, which converts soluble fibrinogen to fibrin. The insoluble fibrin, along with aggregated platelets, forms intravascular clots.
Inflammatory cytokines, such as IL-1α, IL-1β, and TNF-α, initiate coagulation by activating TF. TF interacts with factor VIIa to form factor VIIa-TF complex, which activates factors X and IX. Activation of coagulation in sepsis has been confirmed by marked increases in thrombin-antithrombin complexes and the presence of D-dimer in plasma, indicating activation of the clotting system and fibrinolysis. [23, 24] Tissue plasminogen activator (t-PA) facilitates conversion of plasminogen to plasmin, a natural fibrinolytic.
Endotoxins increase the activity of inhibitors of fibrinolysis—namely, plasminogen activator inhibitor (PAI-1) and thrombin-activatable fibrinolysis inhibitor (TAFI). levels of protein C and endogenous activated protein C (APC) are also decreased in sepsis. Endogenous APC is an important inhibitor of coagulation cofactors Va and VIIa. Thrombin, via thrombomodulin, activates protein C, which then acts as an antithrombotic in the microvasculature. Endogenous APC also enhances fibrinolysis by neutralizing PAI-1 and accelerating t-PA–dependent clot lysis.
The imbalance among inflammation, coagulation, and fibrinolysis results in widespread coagulopathy and microvascular thrombosis and suppressed fibrinolysis, ultimately leading to multiple organ dysfunction and death. The insidious nature of sepsis is such that microcirculatory dysfunction can occur while global hemodynamic parameters such as blood pressure may remain normal. [25]
Circulatory abnormalities
As noted (see Shock Classification, Terminology, and Staging), septic shock falls under the category of distributive shock, which is characterized by pathologic vasodilation and shunting of blood from vital organs to nonvital tissues (eg, skin, skeletal muscle, and fat). The endothelial dysfunction and vascular maldistribution characteristic of distributive shock result in global tissue hypoxia or inadequate delivery of oxygen to vital tissues. In addition, mitochondria can become dysfunctional, thus compromising oxygen utilization at the cellular level.
The predominant hemodynamic feature of septic shock is arterial vasodilation. The mechanisms implicated in this pathologic vasodilation are multifactorial, but the primary factors are thought to be (1) activation of adenosine triphosphate (ATP)-sensitive potassium channels in vascular smooth muscle cells and (2) activation of NO synthase.
The potassium-ATP channels are directly activated by lactic acidosis. NO also activates potassium channels. Potassium efflux from cells results in hyperpolarization, inhibition of calcium influx, and vascular smooth muscle relaxation. [26] The resulting vasodilation can be refractory to endogenous vasoactive hormones (eg, norepinephrine and epinephrine) that are released during shock.
Diminished peripheral arterial vascular tone may cause blood pressure to be dependent on cardiac output, so that vasodilation results in hypotension and shock if insufficiently compensated by a rise in cardiac output. Early in septic shock, the rise in cardiac output is often limited by hypovolemia and a fall in preload because of low cardiac filling pressures. When intravascular volume is augmented, the cardiac output usually is elevated (hyperdynamic phase of sepsis and shock).
Although cardiac output is elevated, the performance of the heart, reflected by stroke work as calculated from stroke volume and blood pressure, is usually depressed. Factors responsible for myocardial depression of sepsis include myocardial depressant substances, coronary blood flow abnormalities, pulmonary hypertension, various cytokines, NO, and beta-receptor downregulation.
Although cardiac output is elevated, the arterial−mixed venous oxygen difference is usually narrow, and the blood lactate level is elevated. This implies that low global tissue oxygen extraction is the mechanism that may limit total body oxygen uptake in septic shock. The basic pathophysiologic problem seems to be a disparity between oxygen uptake and oxygen demand in the tissues, which may be more pronounced in some areas than in others.
This disparity is termed maldistribution of blood flow, either between or within organs, with a resultant defect in the capacity for local extraction of oxygen. During a fall in the oxygen supply, cardiac output becomes distributed so that the most vital organs, such as the heart and brain, remain relatively better perfused than nonvital organs are. However, sepsis leads to regional changes in oxygen demand and regional alteration in the blood flow of various organs.
The peripheral blood flow abnormalities result from the balance between local regulation of arterial tone and the activity of central mechanisms (eg, the autonomic nervous system). Regional regulation and the release of vasodilating substances (eg, NO and prostacyclin) and vasoconstricting substances (eg, endothelin) affect regional blood flow. Increased systemic microvascular permeability also develops, remote from the infectious focus, and contributes to edema of various organs (eg, the lung microcirculation) and to the development of ARDS.
In patients experiencing septic shock, oxygen delivery is relatively high, but the global oxygen extraction ratio is relatively low. Oxygen uptake increases with rising body temperature despite a fall in oxygen extraction.
In patients with sepsis who have low oxygen extraction and elevated arterial lactate levels, oxygen uptake depends on oxygen supply over a much wider range than normal. Therefore, oxygen extraction may be too low for tissue needs at a given oxygen supply, and oxygen uptake may increase with a boost in oxygen supply—a phenomenon termed oxygen uptake supply dependence or pathologic supply dependence. This concept is controversial, however; some investigators argue that supply dependence is an artifact rather than a real phenomenon.
Maldistribution of blood flow, disturbances in the microcirculation, and, consequently, peripheral shunting of oxygen are responsible for diminished oxygen extraction and uptake, pathologic supply dependency of oxygen, and lactate acidemia in patients experiencing septic shock.
Mechanisms of organ dysfunction
Sepsis is described as an autodestructive process that permits the extension of the normal pathophysiologic response to infection (involving otherwise normal tissues), resulting in MODS. Organ dysfunction or organ failure may be the first clinical sign of sepsis, and no organ system is immune to the consequences of the inflammatory excesses of sepsis.
The precise mechanisms of cell injury and resulting organ dysfunction in patients with sepsis are not fully understood. MODS is associated with widespread endothelial and parenchymal cell injury occurring via the following proposed mechanisms:
-
Hypoxic hypoxia – The septic circulatory lesion disrupts tissue oxygenation, alters the metabolic regulation of tissue oxygen delivery, and contributes to organ dysfunction; microvascular and endothelial abnormalities contribute to the septic microcirculatory defect in sepsis; ROS, lytic enzymes, vasoactive substances (eg, NO), and endothelial growth factors lead to microcirculatory injury, which is compounded by the inability of the erythrocytes to navigate the septic microcirculation
-
Direct cytotoxicity – Endotoxin, TNF-α, and NO may cause damage to mitochondrial electron transport, leading to disordered energy metabolism; this is called cytopathic or histotoxic anoxia (ie, inability to use oxygen even when it is present)
-
Apoptosis (programmed cell death) – This is the principal mechanism by which dysfunctional cells are normally eliminated; the proinflammatory cytokines may delay apoptosis in activated macrophages and neutrophils, but other tissues, such as the gut epithelium, may undergo accelerated apoptosis; therefore, derangement of apoptosis plays a critical role in tissue injury in patients with sepsis
-
Immunosuppression – Interaction between proinflammatory and anti-inflammatory mediators may lead to an imbalance and an inflammatory reaction, immunodeficiency may predominate, or both may occur
-
Coagulopathy – Subclinical coagulopathy signified by mild elevation of the thrombin time or activated partial thromboplastin time (aPTT) or by a moderate reduction in platelet count is extremely common, but overt DIC is rare; coagulopathy is caused by deficiencies of coagulation system proteins, including protein C, antithrombin III, and TF inhibitors
Cardiovascular dysfunction
Significant derangement in the autoregulation of the circulatory system is typical in patients with sepsis. Vasoactive mediators cause vasodilatation and increase the microvascular permeability at the site of infection. NO plays a central role in the vasodilation of septic shock. Impaired secretion of vasopressin may also occur, which may permit the persistence of vasodilatation.
Changes in both systolic and diastolic ventricular performance occur in patients with sepsis. Through the Frank-Starling mechanism, cardiac output is often increased to maintain blood pressure in the presence of systemic vasodilatation. Patients with preexisting cardiac disease are unable to increase their cardiac output appropriately.
Because sepsis interferes with the normal distribution of systemic blood flow to organ systems, core organs may not receive appropriate oxygen delivery. The microcirculation is the key target organ for injury in patients with sepsis. A decrease in the number of functional capillaries leads to an inability to extract oxygen maximally; this inability is caused by intrinsic and extrinsic compression of capillaries and plugging of the capillary lumen by blood cells. Increased endothelial permeability leads to widespread tissue edema involving protein-rich fluid.
Hypotension is caused by the redistribution of intravascular fluid volume that results from reduced arterial vascular tone, diminished venous return from venous dilation, and release of myocardial depressant substances.
Pulmonary dysfunction
The pathogenesis of sepsis-induced ARDS is a pulmonary manifestation of SIRS. A complex interaction between humoral and cellular mediators, inflammatory cytokines and chemokines, is involved in this process. A direct or indirect injury to the endothelial and epithelial cells of the lung increases alveolar capillary permeability, causing ensuing alveolar edema. The edema fluid is protein-rich; the ratio of alveolar fluid edema to plasma is 0.75-1.0, whereas in patients with hydrostatic cardiogenic pulmonary edema, the ratio is less than 0.65.
Injury to type II pneumocytes decreases surfactant production; furthermore, the plasma proteins in alveolar fluid inactivate the surfactant previously manufactured. These enhance the surface tension at the air-fluid interfaces, producing diffuse microatelectasis.
Neutrophil entrapment within the pulmonary microcirculation initiates and amplifies the injury to alveolar capillary membrane. ARDS is a frequent manifestation of these effects.
ALI (mild ARDS in the Berlin Definition) is a type of pulmonary dysfunction secondary to parenchymal cellular damage that is characterized by endothelial cell injury and destruction, deposition of platelet and leukocyte aggregates, destruction of type I alveolar pneumocytes, an acute inflammatory response through all injury phases, and repair and hyperplasia of type II pneumocytes. Migration of macrophages and neutrophils into the interstitium and alveoli produces various mediators that contribute to the alveolar and epithelial cell damage.
If addressed at an early stage, ALI may be reversible, but in many cases, the host response is uncontrolled, and ALI progresses to more severe ARDS. Continued infiltration occurs with neutrophils and mononuclear cells, lymphocytes, and fibroblasts. An alveolar inflammatory exudate persists, and type II pneumocyte proliferation is evident. If this process can be halted, complete resolution may occur. In other patients, progressive respiratory failure and pulmonary fibrosis develop.
The central pathologic finding in ARDS is severe injury to the alveolocapillary unit. After initial extravasation of intravascular fluid, inflammation and fibrosis of pulmonary parenchyma develop into a morphologic picture termed diffuse alveolar damage (DAD). The clinical and pathologic evolution can be categorized into the following 3 overlapping phases [27] :
-
Exudative phase (edema and hemorrhage)
-
Proliferative phase (organization and repair)
-
Fibrotic phase (end-stage fibrosis)
The exudative phase of DAD occurs in the first week and is dominated by alveolar edema and hemorrhage (see the images below). Other histologic features include dense eosinophilic hyaline membranes and disruption of the capillary membranes. Necrosis of endothelial cells and type I pneumocytes occur, along with leukoagglutination and deposition of platelet fibrin thrombi.
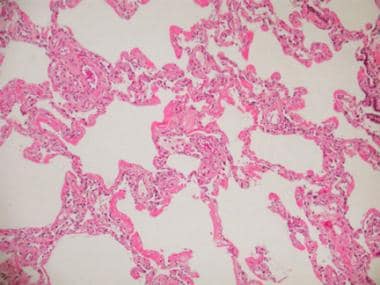 Acute respiratory distress syndrome (ARDS), commonly observed in septic shock as a part of multiorgan failure syndrome, results in pathologically diffuse alveolar damage (DAD). This photomicrograph shows early stage (exudative stage) DAD.
Acute respiratory distress syndrome (ARDS), commonly observed in septic shock as a part of multiorgan failure syndrome, results in pathologically diffuse alveolar damage (DAD). This photomicrograph shows early stage (exudative stage) DAD.
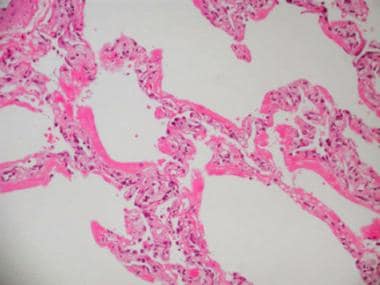 Acute respiratory distress syndrome (ARDS), commonly observed in septic shock as a part of multiorgan failure syndrome, results in pathologically diffuse alveolar damage (DAD). This is a high-powered photomicrograph of early stage (exudative stage) DAD.
Acute respiratory distress syndrome (ARDS), commonly observed in septic shock as a part of multiorgan failure syndrome, results in pathologically diffuse alveolar damage (DAD). This is a high-powered photomicrograph of early stage (exudative stage) DAD.
The proliferative phase is prominent in the second and third week after the onset of ARDS, but it may begin as early as day 3. Organization of the intra-alveolar and interstitial exudate, infiltration with chronic inflammatory cells, parenchymal necrosis, and interstitial myofibroblast reaction occur. Proliferation of type II cells and fibroblasts, which convert the exudate to cellular granulation tissue, is noted, as is excessive collagen deposition, transforming into fibrous tissue (see the images below).
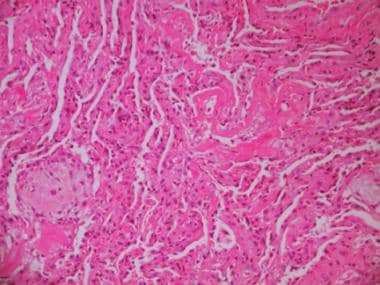 Photomicrograph showing delayed stage (proliferative or organizing stage) of diffuse alveolar damage (DAD). Proliferation of type II pneumocytes has occurred; hyaline membranes as well as collagen and fibroblasts are present.
Photomicrograph showing delayed stage (proliferative or organizing stage) of diffuse alveolar damage (DAD). Proliferation of type II pneumocytes has occurred; hyaline membranes as well as collagen and fibroblasts are present.
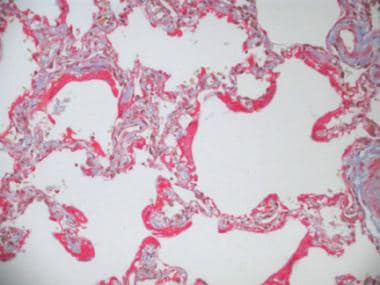 Photomicrograph showing delayed stage (proliferative or organizing stage) of diffuse alveolar damage (DAD). Fibrin stain depicts collagenous tissue, which may develop into fibrotic stage of DAD.
Photomicrograph showing delayed stage (proliferative or organizing stage) of diffuse alveolar damage (DAD). Fibrin stain depicts collagenous tissue, which may develop into fibrotic stage of DAD.
The fibrotic phase occurs by the third or fourth week after the onset of ARDS, though it may begin as early as the first week. The collagenous fibrosis completely remodels the lung, the air spaces are irregularly enlarged, and alveolar duct fibrosis is apparent. Lung collagen deposition increases, and microcystic honeycomb formation and traction bronchiectasis follow.
Gastrointestinal dysfunction
The gastrointestinal (GI) tract may help to propagate the injury of sepsis. Overgrowth of bacteria in the upper GI tract may be aspirated into the lungs and produce nosocomial pneumonia. The gut’s normal barrier function may be affected, thereby allowing translocation of bacteria and endotoxin into the systemic circulation and extending the septic response.
Septic shock usually causes ileus, and the use of narcotics and sedatives delays the institution of enteral feeding. This interferes with optimal nutritional intake, in the face of high protein and energy requirements.
Glutamine is necessary for normal enterocyte functioning. Its absence in commercial formulations of total parenteral nutrition (TPN) leads to breakdown of the intestinal barrier and translocation of the gut flora into the circulation. This may be one of the factors driving sepsis. In addition to inadequate glutamine levels, this may lessen the immune response by decreasing leukocyte and natural killer (NK) cell counts, as well as total B-cell and T-cell counts. [28]
Hepatic and renal dysfunction
By virtue of the liver’s role in host defense, the abnormal synthetic functions caused by liver dysfunction can contribute to both the initiation and the progression of sepsis. The hepatic reticuloendothelial system acts as a first line of defense in clearing bacteria and their products; liver dysfunction leads to a spillover of these products into the systemic circulation.
Acute kidney injury (AKI)—previously termed acute renal failure (ARF)—with remarkably little overt tubular necrosis but markedly impaired renal function often accompanies sepsis. The mechanism for sepsis-induced AKI is poorly understood but is associated with systemic hypotension, cytokinemia (eg, TNF), and activation of neutrophils by endotoxins and other peptides, which indirectly and directly contribute to renal tubular injury.
Central nervous system dysfunction
Central nervous system (CNS) involvement in sepsis produces encephalopathy (septic encephalitis) and peripheral neuropathy. The pathogenesis is poorly defined, but it may involve systemic inflammation from either infectious or noninfectious causes, [29] as well as a combination of the effects of hypoxemia, hypotension, hemorrhage, and medications such as sedatives and analgesics. [29, 30]
Etiology of Septic Shock
Regarding the causes of septic shock, most patients who develop sepsis and septic shock have underlying circumstances that interfere with local or systemic host defense mechanisms. Sepsis is seen most frequently in elderly persons and in those with comorbid conditions that predispose to infection, such as diabetes or any immunocompromising disease. Patients may also have genetic susceptibility, making them more prone to developing septic shock from infections that are well tolerated in the general population. [31, 32, 33, 34, 35]
The most common disease states predisposing to sepsis are malignancies, diabetes mellitus, chronic liver disease, and chronic kidney disease. The use of immunosuppressive agents is also a common predisposing factor. In addition, sepsis is a common complication after major surgery, trauma, and extensive burns. Patients with indwelling catheters or devices are also at high risk.
In most patients with sepsis, a source of infection can be identified. The exceptions are patients who are immunocompromised with neutropenia, in whom an obvious source often is not found.
Causative microorganisms
Before the introduction of antibiotics, gram-positive bacteria were the principal organisms that caused sepsis. Subsequently, gram-negative bacteria became the key pathogens causing sepsis and septic shock. Currently, however, the rates of sepsis and septic shock due to gram-positive organisms are rising again because of the more frequent use of invasive procedures and lines in critically ill patients. As a result, gram-positive and gram-negative microorganisms are now about equally likely to be causative pathogens in septic shock. [36, 37, 38, 39]
Respiratory tract and abdominal infections are the most frequent causes of sepsis, followed by urinary tract and soft-tissue infections. [36, 37, 38, 39] Each organ system tends to be infected by a particular set of pathogens (see below).
Lower respiratory tract infections cause septic shock in 35-50% of patients. [36, 37, 38, 39] The following are the common pathogens:
-
Streptococcus pneumoniae
-
Klebsiella pneumoniae
-
Escherichia coli
-
Legionella species
-
Haemophilus species
-
Staphylococcus aureus
-
Pseudomonas species
-
Anaerobes
-
Gram-negative bacteria
Abdominal and GI tract infections cause septic shock in 20-40% of patients. [36, 37, 38, 39] The following are the common pathogens:
-
E coli
-
Enterococcus species
-
Bacteroides fragilis
-
Acinetobacter species
-
Pseudomonas species
-
Enterobacter species
-
Salmonella species
-
Klebsiella species
-
Anaerobes
Urinary tract infections cause septic shock in 10-30% of patients. [36, 37, 38, 39] The following are the common pathogens:
-
E coli
-
Proteus species
-
Klebsiella species
-
Pseudomonas species
-
Enterobacter species
-
Serratia species
-
Enterococcus species
-
Candida species
Infections of the male and female reproductive systems cause septic shock in 1-5% of patients. [36, 37, 38, 39] The following are the common pathogens:
-
Neisseria gonorrhoeae
-
Gram-negative bacteria
-
Streptococci
-
Anaerobes
Soft-tissue infections cause septic shock in 5-10% of patients. [36, 37, 38, 39] The following are the common pathogens:
-
S aureus
-
Staphylococcus epidermidis
-
Streptococci
-
Clostridium species
-
Gram-negative bacteria
-
Anaerobes
-
Fungi
Infections due to foreign bodies cause septic shock in 1-5% of patients. [36, 37, 38, 39] S aureus, S epidermidis, and fungi (eg, Candida species) are the common pathogens.
Miscellaneous infections, such as CNS infections, also cause septic shock in 1-5% of patients. [36, 37, 38, 39] Neisseria meningitidis is a common cause of such infections (see the image below).
Risk factors
Risk factors for sepsis and septic shock include the following:
-
Extremes of age (< 10 years and >70 years)
-
Primary diseases (eg, liver cirrhosis, alcoholism, diabetes mellitus, cardiopulmonary diseases, solid malignancy, and hematologic malignancy)
-
Immunosuppression (eg, from neutropenia, immunosuppressive therapy [eg, in organ and bone marrow transplant recipients], corticosteroid therapy, injection or IV drug use [see the image below], complement deficiencies, asplenia)
-
Major surgery, trauma, burns
-
Invasive procedures (eg, placement of catheters, intravascular devices, prosthetic devices, hemodialysis and peritoneal dialysis catheters, or endotracheal tubes)
-
Previous antibiotic treatment
-
Prolonged hospitalization
-
Underlying genetic susceptibility
Epidemiology
United States statistics
The incidence of sepsis has been growing in recent decades, for reasons that likely include the following:
-
An increasingly elderly population
-
Increased recognition of the disease
-
Increased performance of invasive procedures and organ transplantation
-
Increased use of immunosuppressive agents and chemotherapy
-
Increased use of indwelling lines and devices
-
A rise in chronic diseases such as end-stage renal disease (ESRD) and HIV infection
An analysis of a large sample from major US medical centers reported the incidence of sepsis (at the time, deemed severe sepsis) as 3 cases per 1000 population and 2.26 cases per 100 hospital discharges. [40] Of these patients, 51.1% were admitted to an intensive care unit (ICU), and an additional 17.3% were cared for in an intermediate care or coronary care unit. When analyzed in relation to age, the incidence of sepsis ranged from 0.2 cases per 1000 admissions in children to 26.2 per 1000 in individuals older than 85 years.
In this analysis, mortality was 28.6% overall, ranging from 10% in children to 38.4% in elderly people. [40] Hospital billing codes were used to identify patients with infection and organ dysfunction consistent with the definition of severe sepsis at that time. Sepsis resulted in an average cost of $22,100 per case, with an annual total cost of $16.7 billion nationally.
In a large retrospective analysis, the National Center for Health Statistics used the National Hospital Discharge Survey of 500 nonfederal US hospitals (which included more than 10 million cases of sepsis over a 22-year period) to report that septicemia accounted for 1.3% of all hospitalizations. [41] The incidence of sepsis increased 3-fold between 1979 and 2000, from 83 cases per 100,000 population per year to 240 per 100,000.
A subsequent large survey of emergency department (ED) visits showed that severe sepsis accounted for more than 500,000 such visits annually (0.7% of total visits), that the majority of patients presented to EDs without an academic affiliation, and that the mean length of stay in the ED was approximately 5 hours. [42]
In a later report, the US Centers for Disease Control and Prevention (CDC) determined that the inflation-adjusted aggregate cost for the treatment of hospital patients with sepsis increased by 12% per year from 1997 to 2008. [43]
In a 2013 report, Gaieski et al showed that in a large population database, the use of different epidemiologic methodologies affects the average annual incidence of severe sepsis, which can vary as much as 3.5-fold, depending on the method utilized. [44] The investigators found that when the codes for sepsis in the International Classification of Diseases, Ninth Revision (ICD-9), were used, the incidence doubled over a 6-year period (2004-2009).
It is possible that the higher incidence rates in this study, relative to those cited in previous studies, may be attributable to the growing awareness of sepsis, the increased use of its code classification, and the inclusion of both ICU and non-ICU patients.
Age-, sex-, and race-related demographics
Sepsis and septic shock occur at all ages. However, a strong correlation exists between advanced age and the incidence of septic shock, with a sharp increase in the number of cases in patients older than 50 years. [40, 45] At present, most sepsis episodes are observed in patients older than 60 years. Advanced age is a risk factor for acquiring nosocomial bloodstream infection (BSI) in the development of severe forms of sepsis.
Overall, compared with younger patients, elderly patients are more susceptible to sepsis, have less physiologic reserve to tolerate the insult from infection, and are more likely to have underlying diseases; all of these factors adversely affect survival. In addition, elderly patients are more likely to have atypical or nonspecific presentations with sepsis.
Epidemiologic data have shown that the age-adjusted incidence and mortality rate are consistently greater in men; the percentage of affected male patients ranges from 52% to 66%. However, it is not clear whether this difference can be attributed to an underlying higher prevalence of comorbid conditions or to a higher incidence of lung infection in men, or whether women are inherently protected against the inflammatory injury that occurs in sepsis. [40, 41]
With regard to ethnicity, one large epidemiologic study showed that the risk of septicemia in the nonwhite population is almost twice that in the white population, with the highest risk accruing to black men. [41] Potential reasons for this difference include issues relating to decreased access to health care and increased prevalence of underlying medical conditions.
Another large epidemiologic study tied the increased incidence in the black population to increased rates of infection necessitating hospitalization and increased development of organ dysfunction. [46] In this study, black patients with septic shock had a higher incidence of underlying diabetes and renal disease, which may explain the higher rates of infection. However, development of acute organ dysfunction was independent of comorbidities. [46]
Mortality Rate
Mortality figures for sepsis and septic shock have commonly been quoted as ranging from 20% to 50%. Clinical trials from the past decade have found the mortality associated with septic shock to range from 24% to 41%. [36, 37, 38, 39] Although one report noted that crude hospital mortality for severe sepsis was significantly lower in the United States (28%) than in Europe (41%), the difference ceased to be significant when adjusted by disease severity. [38]
Important to note, in a 12-year (2000-2012) review of survival from severe sepsis from the Australia and New Zealand ICU database, mortality has decreased from 35% to 18% with decreasing occurrence in all age groups and across all types of hospital settings. These survival improvements are especially important because in this same time span no new sepsis-specific treatments were introduced, suggesting that improved overall quality of care was able to reduce sepsis mortality by half. [47] Thus, studies using a before-and-after design to claim improved sepsis survival are fundamentally flawed because of this nonspecific survival improvement.
Mortality has been found to vary according to the degree of illness, which may range along a spectrum extending from sepsis to septic shock. The following clinical characteristics are related to the severity of sepsis:
-
Abnormal host response to infection
-
Site and type of infection
-
Timing and type of antimicrobial therapy
-
Offending organism
-
Development of shock
-
Any underlying disease
-
Patient’s long-term health condition
-
Location of the patient at the time of septic shock onset
-
Host immunogenetic variation
Factors consistently associated with increased mortality in sepsis include advanced age, comorbid conditions, and clinical evidence of organ dysfunction. [40, 45] One study found that in the setting of suspected infection, simply meeting SIRS criteria, without evidence of organ dysfunction, did not predict increased mortality; this finding suggests that organ dysfunction is a better predictor than SIRS criteria alone. [45] However, there is evidence that meeting greater numbers of SIRS criteria is associated with increased mortality. [48]
Notably, tachypnea is the SIRS criterion that best predicts an adverse outcome. This is likely because tachypnea is also an indicator of pulmonary organ dysfunction and a feature commonly associated with pneumonia and ARDS, both of which are associated with increased mortality in sepsis. Altered mental status is considered a sign of organ dysfunction and is also associated with increased mortality.
In one epidemiologic study, reported mortality figures were 7% for SIRS, 16% for sepsis, 20% for severe sepsis, and 46% for septic shock. [49] Poor prognostic factors included the following:
-
Advanced age
-
Infection with a resistant organism
-
Impaired host immune status
-
Poor prior functional status
-
Continued need for vasopressors past 24 hours
-
Development of sequential organ failure, despite adequate supportive measures and antimicrobial therapy
A link between impaired adrenal function and higher septic shock mortality has been suggested. The adrenal gland is enlarged in these patients as compared with control subjects. A study by Jung et al found that the absence of this enlargement, indicated by total adrenal volume of less than 10 cm3, was associated with increased 28-day mortality in patients with septic shock. [50]
A multicenter prospective study published by Brun-Buisson et al reported a mortality of 56% during ICU stays and 59% during hospital stays, [3] with 27% of all deaths occurring within 2 days of the onset of severe sepsis and 77% occurring within the first 14 days. The risk factors for early mortality in this study were as follows:
-
Higher severity of illness score
-
Acute failure of 2 or more organ systems at the time of sepsis
-
Shock
-
Low blood pH (< 7.3)
Studies have shown that appropriate selection and early administration of antibiotics (ie, antibiotics that are effective against the organism that is ultimately identified) lead to a significant reduction in mortality. [51] For this reason, it is important to initiate broad-spectrum coverage until the specific organism is cultured and antibiotic sensitivities are determined.
Although mortality is known to be high, the effect of sepsis on survivors’ quality of life of survivors has not been well characterized until comparatively recently. It is increasingly evident that septic shock is often a major sentinel event that has lasting effects on the patient’s independence, reliance on family support, and need for long-term nursing home or institutionalized care. [52]
Prolonged tissue hypoperfusion can lead to long-term neurologic and cognitive sequelae. [14] Newer evidence shows that septic shock in elderly persons leads to significant long-term cognitive and functional disability in comparison with hospitalized individuals who have nonsepsis conditions.
-
Strawberry tongue in a child with staphylococcal toxic shock syndrome. Reproduced with permission from Drage, LE. Life-threatening rashes: dermatologic signs of four infectious diseases. Mayo Clin Proc. 1999;74:68-72.
-
Venn diagram showing the overlap of infection, bacteremia, sepsis, systemic inflammatory response syndrome (SIRS), and multiorgan dysfunction.
-
A 26-year-old woman developed rapidly progressive shock associated with purpura and signs of meningitis. Her blood culture results confirmed the presence of Neisseria meningitidis. The skin manifestation seen in this image is characteristic of severe meningococcal infection and is called purpura fulminans.
-
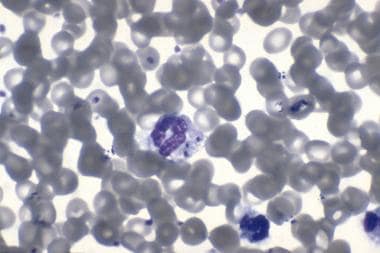
Gram stain of blood showing the presence of Neisseria meningitidis.
-
Acute respiratory distress syndrome (ARDS), commonly observed in septic shock as a part of multiorgan failure syndrome, results in pathologically diffuse alveolar damage (DAD). This photomicrograph shows early stage (exudative stage) DAD.
-
Acute respiratory distress syndrome (ARDS), commonly observed in septic shock as a part of multiorgan failure syndrome, results in pathologically diffuse alveolar damage (DAD). This is a high-powered photomicrograph of early stage (exudative stage) DAD.
-
Photomicrograph showing delayed stage (proliferative or organizing stage) of diffuse alveolar damage (DAD). Proliferation of type II pneumocytes has occurred; hyaline membranes as well as collagen and fibroblasts are present.
-
Photomicrograph showing delayed stage (proliferative or organizing stage) of diffuse alveolar damage (DAD). Fibrin stain depicts collagenous tissue, which may develop into fibrotic stage of DAD.
-
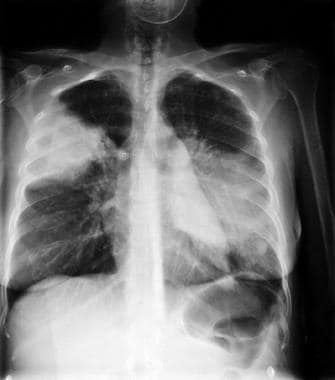
Acute respiratory distress syndrome (ARDS) in a patient who developed septic shock secondary to toxic shock syndrome.
-
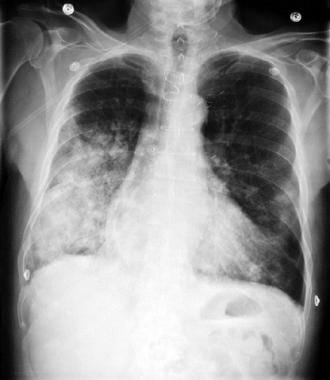
Bilateral airspace disease and acute respiratory failure in a patient with gram-negative septic shock. The source of the sepsis was urosepsis.
-
A 45-year-old woman was admitted to the intensive care unit with septic shock secondary to spontaneous biliary peritonitis. She subsequently developed acute respiratory distress syndrome (ARDS) and multiorgan failure.
-
An 8-year-old boy developed septic shock secondary to Blastomycosis pneumonia. Fungal infections are rare causes of septic shock.
-
A 28-year-old woman who was a former intravenous drug user (human immunodeficiency virus [HIV] status: negative) developed septic shock secondary to bilateral pneumococcal pneumonia.
-
Diagram depicting the pathogenesis of sepsis and multiorgan failure. DIC = disseminated intravascular coagulation; IL = interleukin.
-
Soft-tissue infection secondary to group A streptococci, leading to toxic shock syndrome.
-
Necrotizing cellulitis of toxic shock syndrome.
-
Necrosis of the little toe of the right foot and cellulitis of the foot secondary to group A streptococcal infection.
-
Group A streptococci cause beta hemolysis on blood agar.
-
Gram stain of blood showing group A streptococci that was isolated from a patient who developed toxic shock syndrome. Image courtesy of T. Matthews.
-
A 46-year-old man presented with nonnecrotizing cellulitis and streptococcal toxic shock syndrome. The leg was incised to exclude underlying necrotizing infection. Image courtesy of Rob Green, MD.
-
A 46-year-old man presented with nonnecrotizing cellulitis and streptococcal toxic shock syndrome (same patient as in previous image). This patient also had streptococcal pharyngitis. Image courtesy of Rob Green, MD.
-
A 46-year-old man presented with nonnecrotizing cellulitis and streptococcal toxic shock syndrome (same patient as in previous image). The patient had diffuse erythroderma, a characteristic feature of the syndrome. Image courtesy of Rob Green, MD.
-
A 46-year-old man presented with nonnecrotizing cellulitis and streptococcal toxic shock syndrome (same patient as in previous image). The patient had diffuse erythroderma, a characteristic feature of the syndrome. He improved with antibiotics and intravenous gammaglobulin therapy. Several days later, a characteristic desquamation of the skin occurred over his palms and soles. Image courtesy of Rob Green, MD.
-
Progression of soft-tissue swelling to vesicle or bullous formation is an ominous sign and suggests streptococcal shock syndrome. Image courtesy of S. Manocha.
-
Extensive debridement of necrotizing fasciitis of the hand.
-
Healing of the hand after aggressive surgical debridement of necrotizing fasciitis (same patient as in previous image).
-
A 58-year-old patient presented in septic shock. On physical examination, progressive swelling of the right groin was observed. On exploration, necrotizing cellulitis, but not fasciitis, was present. The wound cultures grew group A streptococci. The patient developed severe shock (toxic shock syndrome). Computed tomography (CT) scanning helped to evaluate the extent of the infection and to exclude other pathologies (eg, psoas abscess, osteomyelitis, inguinal hernia).
-
Computed tomography (CT) scan from a 58-year-old patient who presented in septic shock (same patient as in previous image). Progressive swelling of the right groin was noted, and necrotizing cellulitis, but not fasciitis, was present. The wound cultures grew group A streptococci. The patient developed severe shock (toxic shock syndrome). CT scanning helped in the evaluation of the extent of the infection and in the exclusion of other pathologies (eg, psoas abscess, osteomyelitis, inguinal hernia).
-
Computed tomography (CT) scan from a 58-year-old patient who presented in septic shock (same patient as in previous image). Progressive swelling of the right groin was noted, and necrotizing cellulitis, but not fasciitis, was present. The wound cultures grew group A streptococci. The patient developed severe shock (toxic shock syndrome). CT scanning helped in the evaluation of the extent of the infection and in the exclusion of other pathologies (eg, psoas abscess, osteomyelitis, inguinal hernia).
-
Space-occupying lesion correlating with left temporoparietal metastatic infiltration associated with peritumoral edema.
-
Space-occupying lesion correlating with left temporoparietal metastatic infiltration associated with peritumoral edema (same lesion as shown in previous computed tomography image).
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- General Treatment Guidelines in Septic Shock
- Goals of Hemodynamic Support
- Fluid Resuscitation
- Vasopressor Therapy
- Inotropic Therapy and Augmented Oxygen Delivery
- Empiric Antimicrobial Therapy
- Corticosteroid Therapy
- Glycemic Control
- DVT Prophylaxis and Management of DIC
- Management of Acute Respiratory Distress Syndrome
- Surgical Treatment
- Prevention
- Show All
- Medication
- Questions & Answers
- Media Gallery
- Tables
- References