Practice Essentials
Rhabdomyolysis is a syndrome caused by injury to skeletal muscle and involves leakage of large quantities of potentially toxic intracellular contents into plasma. Its final common pathway may be a disturbance in myocyte calcium homeostasis.
Myoglobin is an important myocyte compound released into plasma (see the image below). After muscle injury, massive plasma myoglobin levels exceed protein binding (of haptoglobin) and can precipitate in glomerular filtrate. Excess myoglobin may thus cause renal tubular obstruction, direct nephrotoxicity (ischemia and tubular injury), intrarenal vasoconstriction, and acute kidney injury (AKI). [1, 2, 3, 4]
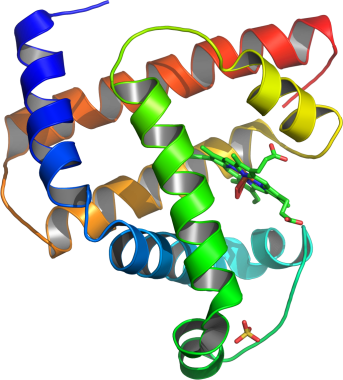 Rhabdomyolysis is associated with the release of myoglobin into plasma. Shown here is a model of helical domains in myoglobin (protein linked to kidney damage in rhabdomyolysis).
Rhabdomyolysis is associated with the release of myoglobin into plasma. Shown here is a model of helical domains in myoglobin (protein linked to kidney damage in rhabdomyolysis).
Signs and symptoms
The classic triad of rhabdomyolysis comprises the following:
-
Myalgias
-
Generalized weakness
-
Darkened urine
In practice, however, presentation varies considerably. Additional nonspecific symptoms include fever, nausea, and vomiting.
In most cases, the history reflects the inciting cause (though in some, it is nonspecific and thus diagnostically unreliable). Possible causes include the following:
-
Alcohol use and resultant unresponsiveness
-
Illicit drug use
-
Use of prescribed medications
-
Heatstroke
-
Infection
-
Trauma
-
Metabolic disorders
-
Inflammatory myopathies
Initial physical findings may be nonspecific. The following may be noted:
-
Muscle pain and tenderness
-
Decreased muscle strength
-
Soft tissue swelling
-
Skin changes consistent with pressure necrosis
-
Hyperthermia, hypothermia, and electrical injuries
-
Crush injuries or deformities in long bones
Complications of rhabdomyolysis include the following:
-
Electrolyte abnormalities
-
Hypoalbuminemia
-
Hyperuricemia
-
Compartment syndrome
-
Acute kidney injury (AKI) and renal failure
-
Disseminated intravascular coagulation (DIC, a late complication)
See Presentation for more detail.
Diagnosis
Laboratory tests that may be useful include the following:
-
Complete blood count (CBC), including hemoglobin, hematocrit, and platelets
-
Serum chemistries
-
Prothrombin time (PT)
-
Activated partial thromboplastin time (aPTT)
-
Serum aldolase
-
Lactate dehydrogenase (LDH)
-
Cardiac troponin I
-
Creatine kinase (most reliable and sensitive indicator of muscle injury)
-
Urine myoglobin
Imaging studies generally play only a small diagnostic role but may be considered in particular settings, as follows:
-
Radiography (suspected fracture)
-
Computed tomography (CT) of the head (altered sensorium, significant head trauma, seizure, neurologic deficits of unknown origin)
-
Magnetic resonance imaging (MRI; assessment of myopathy)
Other tests that may be considered include the following:
-
Electrocardiography (ECG)
-
Measurement of compartment pressures
-
Muscle biopsy
-
Immunoblotting, immunofluorescence, and genetic studies
See Workup for more detail.
Management
Medical management of rhabdomyolysis includes the following:
-
Assessment of the ABCs (A irway, B reathing, C irculation)
-
Identification and correction of the inciting cause (eg, trauma, infection, or toxins)
-
Fluid resuscitation, initiated as soon as possible
-
Institution of measures to prevent of AKI and acute renal failure (ARF) – Urinary alkalization, mannitol, loop diuretics
-
Correction of electrolyte, acid-base, and metabolic abnormalities
Other medical treatment measures may include the following:
-
Dialysis, if indicated
-
Use of free-radical scavengers and antioxidants (clinical utility remains unclear)
-
Discontinuance of any inciting myotoxic agents
Depending on the cause of rhabdomyolysis, surgical care may be necessary, as follows:
-
When intracompartmental pressure exceeds 30 mm Hg, a fasciotomy is advocated
-
Limb fractures may call for surgical and orthopedic treatment
Lifestyle-related treatment measures may be considered, as follows:
-
Dietary modification to help address metabolic disorders or inborn errors of metabolism
-
Avoidance of strenuous activities if such activities cause recurrent myalgias, myopathy, or rhabdomyolysis
-
Maintenance of proper hydration during athletic exertion
-
Prompt attention to indicators of heat exhaustion during hot and humid conditions
See Treatment and Medication for more detail.
Background
Rhabdomyolysis (literally, “dissolution of skeletal muscle”) is a syndrome caused by injury to skeletal muscle and involves leakage of large quantities of potentially toxic intracellular contents into plasma. [5] First described in the victims of crush injury during World War II, [6] it is a final pathway of diverse processes and insults. [7] The final common pathway of rhabdomyolysis may be a disturbance in myocyte calcium homeostasis. [4]
In adults, rhabdomyolysis is characterized by the triad of muscle weakness, myalgias, and dark urine. [8] In many children with this condition, however, all 3 symptoms may not be seen together. [9, 10] Myalgias and generalized muscle weakness are the most common presenting symptoms. Life-threatening renal failure and disseminated intravascular coagulation (DIC) are dreaded complications that appear to be more common in adults. [2]
Rhabdomyolysis has many etiologies and is often multifactorial in adult patients. Infection and inherited disorders appear to be the most prevalent etiologies in children. [11] The physician must be alert to the diagnosis of rhabdomyolysis and to its subtle presentation to prevent acute renal failure. Sensitive laboratory markers of myocyte injury include elevated plasma creatine kinase (CK) levels (often in excess of 4- to 5-fold the upper limit of normal). [11]
Management of rhabdomyolysis consists primarily of correction of fluid and electrolyte anomalies. With adequate supportive measures, the clinical outcome of rhabdomyolysis is often favorable in children. [7] Recurrent episodes of rhabdomyolysis, especially in children, may indicate underlying defects of muscle structure or metabolism. [4]
Pathophysiology
The multiplicity of potential causes of rhabdomyolysis notwithstanding, the final common denominator appears to be disruption of the sarcolemma and release of intracellular myocyte components. Mechanisms of cell destruction in rhabdomyolysis include cellular membrane injury, muscle cell hypoxia, adenosine triphosphate (ATP) depletion, electrolyte disturbances that cause perturbation of sodium-potassium pumps, and generation of oxidative free radicals. [7]
The sarcolemma, a thin membrane that encloses striated muscle fibers, contains numerous pumps that regulate cellular electrochemical gradients. The intercellular sodium concentration is normally maintained at 10 mEq/L by a sodium-potassium adenosine triphosphatase (Na/K-ATPase) pump located in the sarcolemma. [12]
The Na/K-ATPase pump actively transports sodium from the interior of the cell to the exterior. As a result, the interior of the cell is more negatively charged than the exterior because positive charges are transported across the membrane. The gradient pulls sodium to the interior of the cell in exchange for calcium through a protein carrier exchange mechanism. In addition, an active calcium exchanger promotes calcium entry into the sarcoplasmic reticulum and mitochondria.
These processes depend on ATP as a source of energy. ATP depletion appears to be the end result of most causes of rhabdomyolysis. This depletion disrupts cellular transport mechanisms and alters electrolyte composition. [13]
An increase in intracellular calcium levels results in hyperactivity of proteases and proteolytic enzymes and generation of free oxygen radicals. These enzymes and substances increasingly degrade myofilaments and injure membrane phospholipid with leakage of intracellular contents into plasma. These contents include potassium, phosphate, CK, urate, and myoglobin.
Excess fluid may also accumulate within affected muscle tissue. The action of phospholipases in insect and snake venom may cause hemolysis, muscle damage, endothelial necrosis, rhabdomyolysis, and acute kidney injury (AKI). [14] Additionally, muscle damage is amplified by infiltration of activated neutrophils. An inflammatory cascade and reperfusion injury sustains muscle damage and degeneration. [15, 12]
Myoglobin is an important myocyte compound released into plasma (see the image below). After muscle injury, massive plasma myoglobin levels exceed protein binding (of haptoglobin) and can precipitate in glomerular filtrate. Excess myoglobin may thus cause renal tubular obstruction, direct nephrotoxicity (ischemia and tubular injury), intrarenal vasoconstriction, and acute kidney injury (AKI; see below). [2, 3, 4]
 Rhabdomyolysis is associated with the release of myoglobin into plasma. Shown here is a model of helical domains in myoglobin (protein linked to kidney damage in rhabdomyolysis).
Rhabdomyolysis is associated with the release of myoglobin into plasma. Shown here is a model of helical domains in myoglobin (protein linked to kidney damage in rhabdomyolysis).
Acute kidney injury
AKI is believed to be due to decreased extracellular volume, which results in renal vasoconstriction. It is also believed to be due to ferrihemate, which is formed from myoglobin at a pH level of 5.6 or less. Ferrihemate produces free hydroxy radicals and causes direct nephrotoxicity, often through lipid peroxidation. These heme-proteins may enhance vasoconstriction through interactions with nitric oxide (NO) and endothelin receptors. The roles of cytokines in this process have also been discussed. [15]
Renal vasoconstriction and ischemia deplete tubular ATP formation and enhance tubular cell damage. Myoglobin precipitation in renal tubules causes formation of obstructive casts. AKI rarely occurs in patients with chronic myopathies unless it is triggered by a second inciting event. [4] The risk of renal injury is low when initial CK levels are lower than 15,000-20,000 U/L. Lower CK levels may lead to renal injury in patients with sepsis, dehydration, or acidosis. [4]
Gastrointestinal (GI) ischemia is common in patients with fluid and electrolyte imbalances. This ischemia leads to endotoxin absorption, cytokine production, and perpetuation of the systemic inflammatory response.
Etiology
Trauma and muscle compression
Trauma and muscle compression are believed to cause rhabdomyolysis through direct injury to muscle, resulting in disruption of the sarcolemma and direct leakage of cell contents. [2, 15] Occlusion of muscular vessels due to thromboemboli, traumatic injury, or surgical clamping may lead to rhabdomyolysis if muscle tissue ischemia is prolonged. This is the leading cause of rhabdomyolysis in children aged 9-18 years, according to one review. [7]
Orthopedic trauma, including compartment syndromes and fractures, may result in rhabdomyolysis. Such trauma commonly occurs in traffic and occupational accidents. Orthopedic injuries in natural disasters (eg, earthquakes) are compounded by immobilization, hypovolemia, and significant rates of rhabdomyolysis.
Trauma-related events that are particularly likely to lead to rhabdomyolysis include the following:
-
High-voltage electrical injury due to lightning strikes or accidental exposures [2]
-
Heat stroke
-
Extensive burns
-
Near-drowning
-
Prolonged immobilization (eg, after excess alcohol or drug consumption, after an unwitnessed incapacitating stroke, or after prolonged surgical procedures)
Infection
Researchers believe that viruses may cause rhabdomyolysis by directly attacking the muscle and generating muscle-specific toxin. Although infections may lead to only 5% of adult rhabdomyolysis events, viral-induced myositis appears to be the most common etiology for rhabdomyolysis in children younger than age 9 years. [5, 7, 19, 20] Viral infectious disease agents that may cause rhabdomyolysis include the following [21] :
-
Influenza types A and B (most common)
-
Coxsackievirus [23]
-
Echovirus
-
Adenovirus
-
Herpes simplex virus
-
Parainfluenza virus
-
West Nile virus [25]
Cases of rhabdomyolysis related to coronavirus disease 2019 (COVID-19) have been reported. [26, 27, 28]
Legionella is the bacterium classically associated with rhabdomyolysis in adult patients. The pathogenesis is believed to be due to direct invasion and toxic degeneration of muscle fibers. However, any microbe that causes sepsis and toxic shock may potentiate muscle damage and necrosis. Malaria due to Plasmodium falciparum is a common cause of rhabdomyolysis outside the United States. Bacterial infectious agents that may cause rhabdomyolysis include the following [19] :
-
Streptococcus pneumoniae
-
Group B beta-hemolytic streptococci
-
Streptococcus pyogenes
-
Staphylococcus epidermidis
-
Escherichia coli
-
Borrelia burgdorferi
-
Clostridium perfringens
-
Clostridium tetani
-
Viridans streptococci
-
Plasmodium species
-
Rickettsia species
-
Salmonella species [15]
-
Listeria species
-
Legionella species [30]
-
Mycoplasma species [31]
-
Vibrio species
-
Brucella species
-
Bacillus species
-
Leptospira species [32]
Fungal infectious agents that may cause rhabdomyolysis include the following [19] :
-
Candida species
-
Aspergillus species
Metabolic and genetic factors
Certain genetic muscle defects are believed to cause rhabdomyolysis because of the muscle’s inability to use ATP appropriately. Because of inadequate ATP production, the mismatch of energy supply and demand may result in the disruption of cell membranes during exercise.
Any inherited condition that impairs energy delivery to muscle may cause rhabdomyolysis. [33] Such conditions include diseases of glucose, glycogen, fatty acid, or nucleoside metabolism. [34, 35] These disorders often appear in childhood and should be suspected in recurrent cases of myoglobinuria, rhabdomyolysis, or both. Physical exertion and fasting states may exacerbate muscle damage in these disorders. [13, 36]
Electrolyte derangement such as hypophosphatemia is believed to cause rhabdomyolysis because of the resulting shortage of phosphate necessary for the production of ATP. Hypokalemia creates a negative potassium balance, which causes rhabdomyolysis. [37] Hypokalemia due to dehydration and exercise may also cause rhabdomyolysis. [38] Hyponatremia [39] and hypernatremia have also been associated with rhabdomyolysis.
Hypothyroidism, hyperthyroidism, [40] diabetic ketoacidosis and nonketotic hyperosmolar diabetic coma have been associated with rhabdomyolysis. Metabolic and genetic deficiencies that may cause rhabdomyolysis include the following:
-
Glycogen phosphorylase deficiency type V (ie, McArdle disease)
-
Phosphofructokinase deficiency
-
Phosphoglycerate mutase deficiency
-
Phosphoglycerate kinase deficiency (PGK)
-
Carnitine deficiency
-
Carnitine palmityl transferase deficiency (CPT I and II)
-
Mitochondrial respiratory chain enzyme deficiencies (including deficiencies of the acyl-CoA dehydrogenase [ACAD] family of proteins)
-
Myoadenylate deaminase deficiency
Some of these deficiencies are treatable with dietary modification. [7, 15]
Case reports of rhabdomyolysis related to anesthesia in children are believed to be due to underlying muscle disease. Conditions that lead to hyperthermia-related rhabdomyolysis include neuroleptic malignant syndrome and malignant hyperthermia. [41]
A pediatric case series described an often fatal, malignant, hyperthermia-like syndrome characterized by rhabdomyolysis during initial presentation of diabetes mellitus in adolescent males. [42] Although these cases resembled hyperglycemic hyperosmolar nonketotic syndrome (HHNS), patient courses were marked by rhabdomyolysis and cardiovascular instability. The underlying etiology of this catastrophic presentation of adolescent diabetes mellitus is unclear.
Rheumatologic disorders which rarely cause rhabdomyolysis include polymyositis, dermatomyositis, Sjögren syndrome, mixed connective tissue disease and systemic lupus erythematosus. Rhabdomyolysis also has been reported in patients with sickle cell anemia and has mistakenly been identified as a pain crisis.
A study by Vivante et al used whole exome sequencing in a cohort of 21 unrelated families with rhabdomyolysis and detected causative mutations in candidate genes in 43% of the families. The study also reported disease-causing mutations in eight of 58 genes (fatty acid metabolism disorders, glycogen metabolism disorders, abnormal skeletal muscle relaxation disorders, and purine metabolism disorders). [43]
A study by Nelson et al found that sickle cell trait was associated with a higher risk of exertional rhabdomyolysis (similar to the risk associated with tobacco use) among 47,944 soldiers who had undergone testing for hemoglobin AS. [44]
Drugs and myotoxins
Any drug that impairs skeletal muscle ATP production or increases energy requirements may cause rhabdomyolysis. [15] Direct drug-induced sarcolemmal injury is often mediated by activation of phospholipase A.
Toxin-mediated rhabdomyolysis may result from substance abuse, in both adults and adolescents, including abuse of the following:
-
Ethanol
-
Methanol
-
Ethylene glycol
-
Isopropanol
-
Heroin
-
Methadone
-
Barbiturates
-
Cocaine
-
Phencyclidine
-
Lysergic acid diethylamide (LSD)
Ethanol causes metabolic derangement through direct toxicity and disruption of the muscle blood supply by immobilization. Ethanol abuse may cause hypophosphatemia and hypokalemia, which are additive causes of rhabdomyolysis. Alcohol withdrawal, along with delirium tremens and seizures, may be additional factors. Patients who overdose on narcotics and sedative-hypnotics often remain immobilized for extended periods and may have pressure necrosis that results in rhabdomyolysis. Cocaine can directly damage muscle tissue by causing vasoconstriction and tissue ischemia.
Rhabdomyolysis may also result from the use of prescription and nonprescription medications, including the following [45] :
-
Antihistamines (particularly in children)
-
Salicylates
-
Caffeine [50]
-
Fibric acid derivatives (eg, bezafibrate, clofibrate, fenofibrate, and gemfibrozil) [51]
-
Neuroleptics/antipsychotics [52]
-
Anesthetic and paralytic agents (the malignant hyperthermia syndrome)
-
Amphotericin B
-
Quinine
-
Corticosteroids
-
Colchicine
-
Theophylline
-
Cyclic antidepressants
-
Selective serotonin reuptake inhibitors (the serotonin syndrome)
-
Aminocaproic acid
-
Phenylpropanolamine (recalled from the US market)
-
Propofol (continuous infusion) [53]
-
Protease inhibitors
-
Newer-generation antiseizure medications [54]
According to a retrospective analysis of FDA records (2004-2009), [48] the most commonly suspected drug exposure in children younger than 10 years was propofol.
Statins, though tolerated by most adult patients, can cause myopathy and, rarely, rhabdomyolysis. [55, 56] They appear to affect ATP production by impairing mitochondrial function. Specific impairments may involve the electron transport chain. Statins may also alter the balance between protein repair and degradation by affecting ubiquitin proteosome pathway gene expression. [57] Other mechanisms of statin myopathy include depletion of isoprenoids and coenzyme Q10.
Statin-related myopathy risk appears higher in adults with complex medical problems and medication use. [58] Statins appear safe when used in children with hypercholesterolemia. [59]
Environmental toxins that may cause rhabdomyolysis include the following:
-
Carbon monoxide [60]
-
Toluene
-
Hemlock herbs from quail – Rhabdomyolysis after the consumption of quail is well known in the Mediterranean region; it occurs as the result of intoxication by hemlock herbs that the quails consume
-
Snake venom, spider (eg, black widow spider) venom, hornet venom, and massive envenomations of Africanized honey bees
Other causes
Exertional activity (eg, marathons, squats, pushups, sit-ups, high-intensity interval training, and other intense repetitive physical exercises) [33] may cause rhabdomyolysis, especially in untrained individuals. Such events often occur under extremely hot or humid conditions and are related to exertional heat stress and heatstroke. [61, 62] Most of these events occur in military recruits and competitive athletes. In a study by Bäcker et al, most of the cases in athletes involved running, including marathons (54.3%), or weightlifting (14.8%). [63]
Cold exposure in addition to heatstroke is an environmental cause of rhabdomyolysis. [64] Factors that increase the risk of exertional rhabdomyolysis and renal failure include the following [65, 66, 67] :
-
Dehydration
-
Use of nutritional supplements
-
Drug use
-
Sickle cell trait
-
Malignant hyperthermia
Rhabdomyolysis as a complication of respiratory failure and status epilepticus or status asthmaticus has been reported. [68] Whether mechanical ventilation, corticosteroids, or neuromuscular blockade are risk factors in this condition is unclear. [69] Rhabdomyolysis may occur after other conditions associated with excessive muscular activity, including severe dystonia, acute psychosis, and excessive computer keyboard use or gaming. [70]
Epidemiology
United States statistics
Rhabdomyolysis is a common condition in adult populations and is understudied in pediatrics. [71, 7] The National Hospital Discharge Survey reports 26,000 cases annually. [71] Most adult cases of rhabdomyolysis are due to abuse of illicit drugs or alcohol, muscular trauma, crush injuries, and myotoxic effects of prescribed drugs. Rhabdomyolysis is found in 24% of adult patients who present to emergency departments (EDs) with cocaine-related conditions.
In a large adult cohort, 60% of cases of rhabdomyolysis had multiple factors. [71] Significant pediatric etiologies include infections, trauma, metabolic conditions, and muscle diseases. In a retrospective review at a tertiary care pediatric center review spanning 10 years, viral myositis accounted for most cases of rhabdomyolysis in patients aged 0-9 years, whereas trauma was the leading diagnosis in patients aged 9-18 years. [7]
The incidence of myoglobin-induced acute kidney injury in adult rhabdomyolysis ranges from 17-35%. This complication was found in 42% of pediatric patients in a small, retrospective cohort study, in 8.7% in a review of nontraumatic causes in children younger than 7 years, [72] and in only 5% in the larger 10-year review mentioned above. [7, 73] Approximately 28-37% of adult patients require short-term hemodialysis. Rhabdomyolysis is believed to be responsible for 5-20% of all adult cases of acute kidney injury. A comparable figure in children is unavailable.
International statistics
Large numbers of patients may develop rhabdomyolysis and renal failure during disasters such as earthquakes. Severe crush injuries and delayed extrication of survivors characterize such events. Organizations such as the International Society of Nephrology have implemented measures to support local agencies in providing life-saving dialysis treatments for patients with rhabdomyolysis. [2]
Age- and sex-related demographics
Rhabdomyolysis is more common in adults, though it may occur in infants, toddlers, and adolescents who have inherited enzyme deficiencies of carbohydrate or lipid metabolism or who have inherited myopathies, such as Duchenne muscular dystrophy and malignant hyperthermia.
The incidence of rhabdomyolysis is higher in males than in females, especially in the subgroups of patients with trauma and inherited enzyme deficiencies.
Prognosis
The overall mortality for patients with rhabdomyolysis is approximately 5%; however, the risk of death for any single patient is dependent on the underlying etiology and any existing comorbid conditions that may be present and may be significantly higher in patients with AKI and extremely elevated CPK levels.
Implementation of the treatment modalities currently used (see Treatment) has reduced morbidity and mortality. In a 10-year retrospective pediatric review, only 13 of 191 (6%) of patients died. Of these 13 patients, 9 presented in cardiopulmonary arrest and could not be resuscitated. [7]
Rapid intervention and appropriate supportive treatment of rhabdomyolysis-related kidney injury and renal failure improve outcomes in traumatic crush injuries. The ability of medical response teams to provide aggressive hydration and dialysis services enhances survival in large-scale natural disasters such as earthquakes. If treatment modalities are implemented early, many patients recover completely.
Complications
Electrolyte abnormalities are prominent features of rhabdomyolysis. Hyperphosphatemia, hyperkalemia, hypocalcemia (early), hypercalcemia (late) hyperuricemia, and hypoalbuminemia have been described. [5, 15]
Hyperkalemia may be a result of both muscle injury and renal insufficiency or failure. This abnormality may cause life-threatening arrhythmias and should be immediately addressed.
Hypocalcemia is another common metabolic abnormality, resulting from deposition of calcium phosphate. It may also be due to a decreased level of 1,25-dihydroxycholecalciferol in patients with renal failure. Severe hypocalcemia may lead to cardiac arrhythmias, muscular contractions, and seizures. These events may further damage affected muscles. Late findings of hypercalcemia may be related of Ca leakage from damaged muscles and poor clearance if the case is complicated by kidney injury.
Hypoalbuminemia results from proteinuria and direct leakage of protein, whereas hyperuricemia is caused by direct damage to muscle and may contribute to renal tubular damage.
Compartment syndrome may be either a complication of or the inciting cause of rhabdomyolysis. If muscle injury has occurred, measure compartment pressures; if the pressure is higher than 30 mm Hg, fasciotomy is indicated. [2]
Acute kidney injury (AKI) occurs in 17-35% of adult patients [74] and in 5-42% in 2 pediatric case series. [7] Etiologies of AKI may be related to hypovolemia, vasoconstriction, and myoglobin toxicity.
AKI and disseminated intravascular coagulation (DIC, a late complication) are the most severe complications of rhabdomyolysis, often developing 12-72 hours after initial muscle damage. AKI may account for as many as 35% of adult cases. This figure may be as low as 5% in children. [7, 71] Rhabdomyolysis may account for 7-10% of acute kidney injuries in the United States. [4, 71]
Renal failure may also develop in patients treated with optimal measures. Mechanisms of renal injury are multifactorial and may include renal vasoconstriction, intraluminal myoglobin cast formation, and heme-protein cellular toxicity. Myoglobin and hemoglobin toxic effect on the glomerulus are enhanced by aciduria and hypovolemia.
Patient Education
Educate patients about the causes of rhabdomyolysis and its prevention. Provide genetic counseling for families with inherited muscle enzyme and energy substrate deficiencies. Educate high-school and college athletes about signs of dehydration and heat-related injuries. Advise patients with rhabdomyolysis caused by hyperthermia or inordinate exertion to exercise in moderation, with careful attention to hydration and external methods of cooling.
Caution patients that intense exertional activity, such as marathons, squats, pushups, sit-ups, and high-intensity interval training, can cause rhabdomyolysis, particularly in untrained individuals. Such episodes often occur under extremely hot or humid conditions and are related to exertional heat stress and heatstroke.
Advise patients with rhabdomyolysis related to ethanol, recreational drugs, or prescription medications to discontinue use of the offending agent. Refer these patients to a rehabilitation program if necessary.
-
Rhabdomyolysis is associated with the release of myoglobin into plasma. Shown here is a model of helical domains in myoglobin (protein linked to kidney damage in rhabdomyolysis).