Practice Essentials
Pulmonary embolism (PE) is when a blood clot (thrombus) becomes lodged in an artery in the lung and blocks blood flow to the lung. Pulmonary embolism usually arises from a thrombus that originates in the deep venous system of the lower extremities; however, it rarely also originates in the pelvic, renal, upper extremity veins, or the right heart chambers (see the image below). After traveling to the lung, large thrombi can lodge at the bifurcation of the main pulmonary artery or the lobar branches and cause hemodynamic compromise.
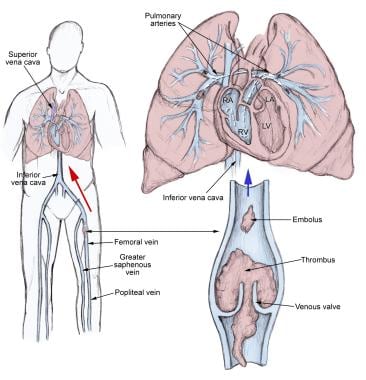 The pathophysiology of pulmonary embolism. Although pulmonary embolism can arise from anywhere in the body, most commonly it arises from the calf veins. The venous thrombi predominately originate in venous valve pockets (inset) and at other sites of presumed venous stasis. To reach the lungs, thromboemboli travel through the right side of the heart. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.
The pathophysiology of pulmonary embolism. Although pulmonary embolism can arise from anywhere in the body, most commonly it arises from the calf veins. The venous thrombi predominately originate in venous valve pockets (inset) and at other sites of presumed venous stasis. To reach the lungs, thromboemboli travel through the right side of the heart. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.
Pulmonary thromboembolism is not a disease in and of itself. Rather, it is a complication of underlying venous thrombosis. Under normal conditions, microthrombi (tiny aggregates of red cells, platelets, and fibrin) are formed and lysed continually within the venous circulatory system.
Signs and symptoms
The classic presentation of PE is the abrupt onset of pleuritic chest pain, shortness of breath, and hypoxia. However, most patients with pulmonary embolism have no obvious symptoms at presentation. Rather, symptoms may vary from sudden catastrophic hemodynamic collapse to gradually progressive dyspnea. The diagnosis of pulmonary embolism should be suspected in patients with respiratory symptoms unexplained by an alternative diagnosis.
Patients with pulmonary embolism may present with atypical symptoms, such as the following:
-
Seizures
-
Syncope
-
Abdominal pain
-
Fever
-
Productive cough
-
Wheezing
-
Decreasing level of consciousness
-
New onset of atrial fibrillation
-
Hemoptysis
-
Flank pain [1]
-
Delirium (in elderly patients) [2]
See Clinical Presentation for more detail.
Diagnosis
Evidence-based literature supports the practice of using clinical scoring systems to determine the clinical probability of pulmonary embolism before proceeding with testing. [3] Validated clinical prediction rules should be used to estimate pretest probability of pulmonary embolism and to interpret test results. [4]
Physical signs of pulmonary embolism include the following:
-
Tachypnea (respiratory rate >20/min): 96%
-
Rales: 58%
-
Accentuated second heart sound: 53%
-
Tachycardia (heart rate >100/min): 44%
-
Fever (temperature >37.8°C [100.04°F]): 43%
-
Diaphoresis: 36%
-
S3 or S4 gallop: 34%
-
Clinical signs and symptoms suggesting thrombophlebitis: 32%
-
Lower extremity edema: 24%
-
Cardiac murmur: 23%
-
Cyanosis: 19%
Testing
Perform diagnostic testing on symptomatic patients with suspected pulmonary embolism to confirm or exclude the diagnosis or until an alternative diagnosis is found. Routine laboratory findings are nonspecific and are not helpful in pulmonary embolism, although they may suggest another diagnosis.
A hypercoagulation workup should be performed if no obvious cause for embolic disease is apparent, including screening for conditions such as the following:
-
Antithrombin III deficiency
-
Protein C or protein S deficiency
-
Lupus anticoagulant
-
Homocystinuria
-
Occult neoplasm
-
Connective tissue disorders
Potentially useful laboratory tests in patients with suspected pulmonary embolism include the following:
-
D-dimer testing
-
Ischemia-modified albumin level
-
White blood cell count
-
Arterial blood gases:
-
Serum troponin levels
-
Brain natriuretic peptide
Imaging studies
Imaging studies that aid in the diagnosis of pulmonary embolism include the following:
-
Computed tomography angiography (CTA): Multidetector-row CTA (MDCTA) is the criterion standard for diagnosing pulmonary embolism
-
Pulmonary angiography: Criterion standard for diagnosing pulmonary embolism when MDCTA is not available
-
Chest radiography: Abnormal in most cases of pulmonary embolism, but nonspecific
-
V/Q scanning: When CT scanning is not available or is contraindicated
-
ECG: Most common abnormalities are tachycardia and nonspecific ST-T wave abnormalities
-
MRI: Using standard or gated spin-echo techniques, pulmonary emboli demonstrate increased signal intensity within the pulmonary artery
-
Echocardiography: Transesophageal echocardiography may identify central pulmonary embolism
-
Venography: Criterion standard for diagnosing DVT
-
Duplex ultrasonography: Noninvasive diagnosis of pulmonary embolism by demonstrating the presence of a DVT at any site
See Workup for more detail.
Management
Anticoagulation and thrombolysis
Immediate full anticoagulation is mandatory for all patients suspected of having DVT or PE. [5] Diagnostic investigations should not delay empirical anticoagulant (blood thinner) therapy.
Thrombolytic therapy should be used in patients with acute pulmonary embolism who have hypotension (systolic blood pressure< 90 mm Hg) who do not have a high bleeding risk and in selected patients with acute pulmonary embolism not associated with hypotension who have a low bleeding risk and whose initial clinical presentation or clinical course suggests a high risk of developing hypotension. [5]
Long-term anticoagulation is critical to the prevention of recurrence of DVT or pulmonary embolism, because even in patients who are fully anticoagulated, DVT and pulmonary embolism can and often do recur.
Anticoagulation medications include the following:
-
Unfractionated heparin
-
Low-molecular-weight heparin
-
Factor Xa inhibitors
-
Fondaparinux
-
Warfarin
Thrombolytic agents used in managing pulmonary embolism include the following:
-
Alteplase
-
Reteplase
Surgical options
Surgical management options include the following:
-
Catheter embolectomy and fragmentation or surgical embolectomy
-
Placement of vena cava filters
See Treatment and Medication for more detail.
Background
Pulmonary embolism (PE) is a common and potentially lethal condition. Most patients who succumb to pulmonary embolism do so within the first few hours of the event. Despite diagnostic advances, delays in pulmonary embolism diagnosis are common and represent an important issue. [6] As a cause of sudden death, massive pulmonary embolism is second only to sudden cardiac death.
In patients who survive a pulmonary embolism, recurrent embolism and death can be prevented with prompt diagnosis and therapy. Unfortunately, the diagnosis is often missed because patients with pulmonary embolism present with nonspecific signs and symptoms. If left untreated, approximately one third of patients who survive an initial pulmonary embolism die from a subsequent embolic episode. (See Prognosis.)
When a pulmonary embolism is identified, it is characterized as acute or chronic. In terms of pathologic diagnosis, an embolus is acute if it is situated centrally within the vascular lumen or if it occludes a vessel (vessel cutoff sign) (see the first image below). Acute pulmonary embolism commonly causes distention of the involved vessel. An embolus is chronic if it is eccentric and contiguous with the vessel wall (see the second image below), it reduces the arterial diameter by more than 50%, evidence of recanalization within the thrombus is present, and an arterial web is present.
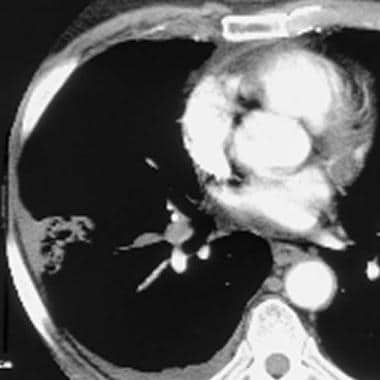 Computed tomography angiogram in a 53-year-old man with acute pulmonary embolism. This image shows an intraluminal filling defect that occludes the anterior basal segmental artery of the right lower lobe. Also present is an infarction of the corresponding lung, which is indicated by a triangular, pleura-based consolidation (Hampton hump).
Computed tomography angiogram in a 53-year-old man with acute pulmonary embolism. This image shows an intraluminal filling defect that occludes the anterior basal segmental artery of the right lower lobe. Also present is an infarction of the corresponding lung, which is indicated by a triangular, pleura-based consolidation (Hampton hump).
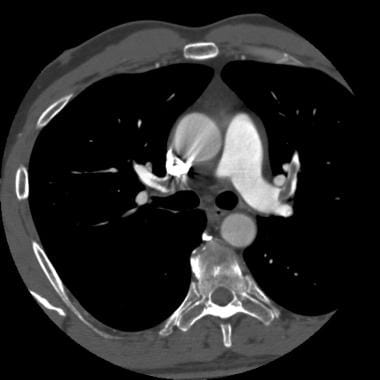 Computed tomography angiography in a young man who experienced acute chest pain and shortness of breath after a transcontinental flight. This image demonstrates a clot in the anterior segmental artery in the left upper lung (LA2) and a clot in the anterior segmental artery in the right upper lung (RA2).
Computed tomography angiography in a young man who experienced acute chest pain and shortness of breath after a transcontinental flight. This image demonstrates a clot in the anterior segmental artery in the left upper lung (LA2) and a clot in the anterior segmental artery in the right upper lung (RA2).
A pulmonary embolism is also characterized as central or peripheral, depending on the location or the arterial branch involved. Central vascular zones include the main pulmonary artery, the left and right main pulmonary arteries, the anterior trunk, the right and left interlobar arteries, the left upper lobe trunk, the right middle lobe artery, and the right and left lower lobe arteries. A pulmonary embolus is characterized as massive when it involves both pulmonary arteries or when it results in hemodynamic compromise. Peripheral vascular zones include the segmental and subsegmental arteries of the right upper lobe, the right middle lobe, the right lower lobe, the left upper lobe, the lingula, and the left lower lobe. (See Physical Examination.)
The variability of presentation sets the patient and clinician up for potentially missing the diagnosis. The challenge is that the "classic" presentation with abrupt onset of pleuritic chest pain, shortness of breath, and hypoxia is rarely seen. Studies of patients who died unexpectedly of pulmonary embolism revealed that the patients had complained of nagging symptoms, often for weeks, before dying. Forty percent of these patients had been seen by a physician in the weeks prior to their death. [7] (See the images below.)
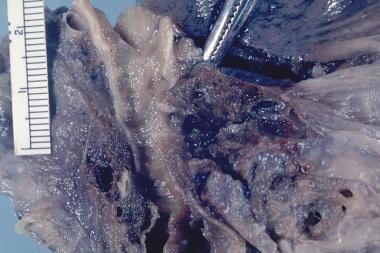 Pulmonary embolism was identified as the cause of death in a patient who developed shortness of breath while hospitalized for hip joint surgery. This is a close-up view.
Pulmonary embolism was identified as the cause of death in a patient who developed shortness of breath while hospitalized for hip joint surgery. This is a close-up view.
The most important conceptual advance regarding pulmonary embolism over the last several decades has been the realization that pulmonary embolism is not a disease; rather, pulmonary embolism is a complication of venous thromboembolism, most commonly deep venous thrombosis (DVT; shown in the image below). Virtually every physician who is involved in patient care encounters patients who are at risk for venous thromboembolism, and therefore at risk for pulmonary embolism. (See Etiology of Pulmonary Embolism.)
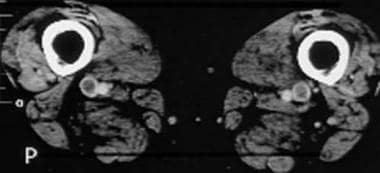 Computed tomography venograms in a 65-year-old man with possible pulmonary embolism. This image shows acute deep venous thrombosis with intraluminal filling defects in the bilateral superficial femoral veins.
Computed tomography venograms in a 65-year-old man with possible pulmonary embolism. This image shows acute deep venous thrombosis with intraluminal filling defects in the bilateral superficial femoral veins.
Clinical signs and symptoms for pulmonary embolism are nonspecific; therefore, patients suspected of having pulmonary embolism—because of unexplained dyspnea, tachypnea, or chest pain or the presence of risk factors for pulmonary embolism—must undergo diagnostic tests until the diagnosis is ascertained or eliminated or an alternative diagnosis is confirmed. Further, routine laboratory findings are nonspecific and are not helpful in pulmonary embolism, although they may suggest another diagnosis. Pulmonary angiography historically was the criterion standard for the diagnosis of pulmonary embolism, but with the improved sensitivity and specificity of CT angiography, it is now rarely performed. (See Workup.)
Immediate full anticoagulation is mandatory for all patients suspected to have DVT or pulmonary embolism. Diagnostic investigations should not delay empirical anticoagulant therapy. (See Treatment.)
Long-term anticoagulation is critical to the prevention of recurrence of DVT or pulmonary embolism. The general consensus is that a significant reduction in recurrence is associated with 3-6 months of anticoagulation. (See Medication.)
Anatomy
Knowledge of bronchovascular anatomy (seen in the image below) is the key to the accurate interpretation of CT scans obtained for the evaluation of pulmonary embolism. A systematic approach in identifying all vessels is important. The bronchovascular anatomy has been described on the basis of the segmental anatomy of lungs. The segmental arteries are seen near the accompanying branches of the bronchial tree and are situated either medially (in the upper lobes) or laterally (in the lower lobes, lingula, and right middle lobe).
 The pathophysiology of pulmonary embolism. Although pulmonary embolism can arise from anywhere in the body, most commonly it arises from the calf veins. The venous thrombi predominately originate in venous valve pockets (inset) and at other sites of presumed venous stasis. To reach the lungs, thromboemboli travel through the right side of the heart. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.
The pathophysiology of pulmonary embolism. Although pulmonary embolism can arise from anywhere in the body, most commonly it arises from the calf veins. The venous thrombi predominately originate in venous valve pockets (inset) and at other sites of presumed venous stasis. To reach the lungs, thromboemboli travel through the right side of the heart. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.
Pulmonary thromboembolism is not a disease in and of itself. Rather, it is a complication of underlying venous thrombosis. Under normal conditions, microthrombi (tiny aggregates of red cells, platelets, and fibrin) are formed and lysed continually within the venous circulatory system. This dynamic equilibrium ensures local hemostasis in response to injury without permitting uncontrolled propagation of clot. (See Etiology of Pulmonary Embolism.)
Pathophysiology
There are both respiratory and hemodynamic consequences associated with pulmonary embolism.
Respiratory consequences
Acute respiratory consequences of PE include the following:
-
Increased alveolar dead space
-
Hypoxemia
-
Hyperventilation
Additional consequences that may occur include regional loss of surfactant and pulmonary infarction (see the image below). Arterial hypoxemia is a frequent, but not universal, finding in patients with acute embolism. The mechanisms of hypoxemia include ventilation-perfusion mismatch, intrapulmonary shunts, reduced cardiac output, and intracardiac shunt via a patent foramen ovale. Pulmonary infarction is an uncommon consequence because of the bronchial arterial collateral circulation.
Hemodynamic consequences
Pulmonary embolism reduces the cross-sectional area of the pulmonary vascular bed, resulting in an increment in pulmonary vascular resistance, which, in turn, increases the right ventricular afterload. If the afterload is increased severely, right ventricular failure may ensue. In addition, the humoral and reflex mechanisms contribute to the pulmonary arterial constriction. Following the initiation of anticoagulant therapy, the resolution of emboli usually occurs rapidly during the first 2 weeks of therapy; however, it can persist on chest imaging studies for months to years. Chronic pulmonary hypertension may occur with failure of the initial embolus to undergo lyses or in the setting of recurrent thromboemboli.
Etiology of Pulmonary Embolism
Three primary influences predispose a patient to blood clot formation; these form the so-called Virchow triad, which consists of the following [8, 9, 10] :
-
Endothelial injury
-
Stasis or turbulence of blood flow
-
Blood hypercoagulability
Thrombosis usually originates as a platelet nidus on valves in the veins of the lower extremities. Further growth occurs by accretion of platelets and fibrin and progression to red fibrin thrombus, which may either break off and embolize or result in total occlusion of the vein. The endogenous thrombolytic system leads to partial dissolution; then, the thrombus becomes organized and is incorporated into the venous wall.
Pulmonary emboli usually arise from thrombi originating in the deep venous system of the lower extremities; however, they may rarely originate in the pelvic, renal, or upper extremity veins or the right heart chambers. After traveling to the lung, large thrombi can lodge at the bifurcation of the main pulmonary artery or the lobar branches and cause hemodynamic compromise. Smaller thrombi typically travel more distally, occluding smaller vessels in the lung periphery. These are more likely to produce pleuritic chest pain by initiating an inflammatory response adjacent to the parietal pleura. Most pulmonary emboli are multiple, and the lower lobes are involved more commonly than the upper lobes.
The causes for pulmonary embolism are multifactorial and are not readily apparent in many cases. The causes described in the literature include the following:
-
Venous stasis
-
Hypercoagulable states
-
Immobilization
-
Surgery and trauma
-
Pregnancy
-
Oral contraceptives and estrogen replacement
-
Malignancy
-
Hereditary factors
-
Acute medical illness
A study by Malek et al confirmed the hypothesis that individuals with HIV infection are more likely to have clinically detected thromboembolic disease. [11] The risk of developing a pulmonary embolism or DVT is increased 40% in these individuals.
Venous stasis
Venous stasis leads to accumulation of platelets and thrombin in veins. Increased viscosity may occur due to polycythemia and dehydration, immobility, raised venous pressure in cardiac failure, or compression of a vein by a tumor.
Hypercoagulable states
The complex and delicate balance between coagulation and anticoagulation is altered by many diseases, by obesity, or by trauma. It can also occur after surgery.
Concomitant hypercoagulability may be present in disease states where prolonged venous stasis or injury to veins occurs.
Hypercoagulable states may be acquired or congenital. Factor V Leiden mutation causing resistance to activated protein C is the most common risk factor. Factor V Leiden mutation is present in up to 5% of the normal population and is the most common cause of familial thromboembolism.
Primary or acquired deficiencies in protein C, protein S, and antithrombin III are other risk factors. Deficiency of these natural blood thinners is responsible for 10% of venous thrombosis in younger people.
Immobilization
Immobilization leads to local venous stasis by accumulation of clotting factors and fibrin, resulting in blood clot formation. The risk of pulmonary embolism increases with prolonged bed rest or immobilization of a limb in a cast.
In the Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) study, immobilization (usually because of surgery) was the risk factor most commonly found in patients with pulmonary embolism.
Surgery and trauma
A prospective study by Geerts and colleagues indicated that major trauma was associated with a 58% incidence of DVT in the lower extremities and an 18% incidence in proximal veins. [12]
Surgical and accidental traumas predispose patients to venous thromboembolism by activating clotting factors and causing immobility. Pulmonary embolism may account for 15% of all postoperative deaths. Leg amputations and hip, pelvic, and spinal surgery are associated with the highest risk.
Fractures of the femur and tibia are associated with the highest risk of fracture-related pulmonary embolism, followed by pelvic, spinal, and other fractures. Severe burns also carry a high risk of DVT or pulmonary embolism.
Pregnancy
The incidence of thromboembolic disease in pregnancy has been reported to range from 1 case in 200 deliveries to 1 case in 1400 deliveries (see Epidemiology). Fatal events are rare, with 1-2 cases occurring per 100,000 pregnancies.
Oral contraceptives and estrogen replacement
Estrogen-containing birth control pills have increased the occurrence of venous thromboembolism in healthy women. The risk is proportional to the estrogen content and is increased in postmenopausal women on hormonal replacement therapy. The relative risk is 3-fold, but the absolute risk is 20-30 cases per 100,000 persons per year.
Malignancy
Malignancy has been identified in 17% of patients with venous thromboembolism. Pulmonary emboli have been reported to occur in association with solid tumors, leukemias, and lymphomas. This is probably independent of the indwelling catheters often used in such patients. [13] The neoplasms most commonly associated with pulmonary embolism, in descending order of frequency, are pancreatic carcinoma; bronchogenic carcinoma; and carcinomas of the genitourinary tract, colon, stomach, and breast.
Hereditary factors
Hereditary factors associated with the development of pulmonary embolism include the following:
-
Antithrombin III deficiency
-
Protein C deficiency
-
Protein S deficiency
-
Factor V Leiden (most common genetic risk factor for thrombophilia)
-
Plasminogen abnormality
-
Plasminogen activator abnormality
-
Fibrinogen abnormality
-
Resistance to activated protein C
Acute medical illness
Acute medical illnesses associated with the development of pulmonary embolism include the following:
-
AIDS (lupus anticoagulant)
-
Behçet disease
-
Congestive heart failure (CHF)
-
Myocardial infarction (heart attack)
-
Polycythemia
-
Systemic lupus erythematosus
-
Ulcerative colitis
Additional risk factors
Risk factors for pulmonary embolism also include the following:
-
Drug abuse (intravenous [IV] drugs)
-
Drug-induced lupus anticoagulant
-
Hemolytic anemias
-
Heparin-associated thrombocytopenia
-
Homocystinemia
-
Homocystinuria
-
Hyperlipidemias
-
Phenothiazines
-
Thrombocytosis
-
Varicose veins
-
Venography
-
Venous pacemakers
-
Venous stasis
-
Warfarin (first few days of therapy)
-
Inflammatory bowel disease
-
Sleep-disordered breathing [14]
In the PIOPED II study, 94% of patients with pulmonary embolism had 1 or more of the following risk factors [15] :
-
Immobilization
-
Travel of 4 hours or more in the past month
-
Surgery within the last 3 months
-
Malignancy, especially lung cancer
-
Current or past history of thrombophlebitis
-
Trauma to the lower extremities and pelvis during the past 3 months
-
Smoking
-
Central venous instrumentation within the past 3 months
-
Stroke, paresis, or paralysis
-
Prior pulmonary embolism
-
Heart failure
-
Chronic obstructive pulmonary disease
Pulmonary embolism in children
In contrast to adults, most children (98%) diagnosed with pulmonary emboli have an identifiable risk factor or a serious underlying disorder (see Epidemiology).
In 1993, David et al reported that 21% of children with DVT and/or pulmonary emboli had an indwelling central venous catheter. [16] Additional series have reported the presence of central lines in as many as 36% of patients. [17] A clot may form as a fibrin sleeve that encases the catheter. When the catheter is removed, the fibrin sleeve is often dislodged, releasing a nidus for embolus formation. In another scenario, a thrombus may adhere to the vessel wall adjacent to the catheter.
David and colleagues also reported that 5-10% of children with venous thromboembolic disease have inherited disorders of coagulation, such as antithrombin III, protein C, or protein S deficiency. [16] In 1997, Nuss et al reported that 70% of children with a diagnosis of pulmonary embolism have antiphospholipid antibodies or coagulation-regulatory protein abnormalities. [18] However, this was a small study in a population with clinically recognized pulmonary emboli; hence, its applicability to the broader pediatric population is uncertain.
A study reported that major thrombosis or pulmonary embolism was present in more than 33% of children treated with long-term hyperalimentation and that pulmonary embolism was the major cause of death in 30% of these children. Fat embolization may exacerbate this clinical picture. [19]
Dehydration, especially hyperosmolar dehydration, is typically observed in younger infants with pulmonary emboli.
Epidemiology
United States statistics
The incidence of pulmonary embolism in the United States is estimated to be 1 case per 1000 persons per year. [20] Studies from 2008 suggest that the increasing use of computed tomography (CT) scanning for assessing patients with possible pulmonary embolism has led to an increase in the reported incidence of pulmonary embolism. [21, 22]
From 1979-1998, the age-adjusted death rate for pulmonary embolism in the United States decreased from 191 deaths per million population to 94 deaths per million population. [20] Regional studies covering the years after 1998 found either a slight decrease in the incidence of mortality or no change in the frequency. [21, 22]
Pulmonary embolism is present in 60-80% of patients with DVT, even though more than half these patients are asymptomatic. Pulmonary embolism is the third most common cause of death in hospitalized patients, with at least 650,000 cases occurring annually. Autopsy studies have shown that approximately 60% of patients who have died in the hospital had pulmonary embolism, with the diagnosis having been missed in up to 70% of the cases. Prospective studies have demonstrated DVT in 10-13% of all medical patients placed on bed rest for 1 week, 29-33% of all patients in medical intensive care units, 20-26% of patients with pulmonary diseases who are given bed rest for 3 or more days, 27-33% of patients admitted to a critical care unit after a myocardial infarction, and 48% of patients who are asymptomatic after a coronary artery bypass graft.
Venous thromboembolism is a major health problem. The average annual incidence of venous thromboembolism in the United States is 1 person per 1000 population, [3, 23, 24] with about 250,000 incident cases occurring annually.
A challenge in understanding the real disease has been that autopsy studies have found an equal number of patients diagnosed with pulmonary embolism at autopsy was were initially diagnosed by clinicians. [23, 25] This has led to estimates of between 650,000 to 900,000 fatal and nonfatal venous thromboembolic events occurring in the US annually. The incidence of venous thromboembolism has not changed significantly over the last 25 years. [23] Capturing the true incidence going forward will be challenging because of the decreasing rate of autopsy. In a longitudinal, 25-year prospective study from 1966-1990, autopsy rates dropped from 55% to 30% over the study period. [23] Current trends would suggest a continued decline in autopsy rate.
International statistics
The incidence of PE may differ substantially from country to country; observed variation is likely due to differences in the accuracy of diagnosis rather than in the actual incidence.
Canadian data derived from 15 tertiary care centers showed a frequency of 0.86 events per 10,000 pediatric hospital admissions for patients aged 1 month to 1 year. [26] Frequency of pulmonary embolism in developed countries has been increasing when compared with historical data. This increase in frequency is linked with the increased use of central venous lines in the pediatric population. [27] The overall frequency in children is still considerably less than that in adults.
Association between sex and pulmonary embolism
Data are conflicting as to whether male sex is a risk factor for pulmonary embolism; however, an analysis of national mortality data found that death rates from pulmonary embolism were 20-30% higher among men than among women. [20] The incidence of venous thromboembolic events in the older population is greater among men than women. In patients younger than 55 years, the incidence of pulmonary is higher in females. The overall age- and sex-adjusted annual incidence of venous thromboembolism is reported to be 117 cases per 100,000 people (DVT, 48 cases per 100,000; pulmonary embolism, 69 cases per 100,000). [23]
A prospective cohort study of female nurses found an association between idiopathic pulmonary embolism and hours spent sitting each week. Women who reported in both 1988 and 1990 that they sat more than 40 hours per week had more than twice the risk of pulmonary embolism compared with women who reported both years that they sat less than 10hours per week. [28]
Association between race and pulmonary embolism
The incidence of pulmonary embolism appears to be significantly higher in blacks than in whites. [29] Mortality rates from pulmonary embolism for blacks have been 50% higher than those for whites, and those for whites have been 50% higher than those for people of other races (eg, Asians, Native Americans). [20] Asian/Pacific Islanders/American Indian patients have a markedly lower risk of thromboembolism. [20, 30]
Pulmonary embolism in elderly persons
Pulmonary embolism is increasingly prevalent among elderly patients, yet the diagnosis is missed more often in these patients than in younger ones because respiratory symptoms often are dismissed as being chronic. Even when the diagnosis is made, appropriate therapy frequently is inappropriately withheld because of bleeding concerns. An appropriate diagnostic workup and therapeutic anticoagulation with a careful risk-to-benefit assessment is recommended in this patient population.
Pulmonary embolism in pediatric patients
DVT and pulmonary embolism are rare in pediatric practice. In 1993, David et al identified 308 children reported in the medical literature from 1975-1993 with DVT of an extremity and/or pulmonary embolism. [16] In 1986, Bernstein reported 78 episodes of pulmonary embolism per 100,000 hospitalized adolescents. [31] Unselected autopsy studies in children estimate the incidence of pulmonary embolism from 0.05-3.7%.
However, among pediatric patients in whom DVT or pulmonary emboli do occur, these conditions are associated with significant morbidity and mortality. Various authors suggest that pulmonary embolism contributes to the death of affected children in approximately 30% of cases. [32] (Others, however, have reported pulmonary embolism as a cause of death in fewer than 5% of affected children. [33] )
Thromboembolic disease in pregnancy
A population-based study covering the years 1966-1995 collated the cases of DVT or pulmonary embolism in women during pregnancy or postpartum. The relative risk was 4.29, and the overall incidence of venous thromboembolism (absolute risk) was 199.7 incidents per 100,000 woman-years. Among postpartum women, the annual incidence was 5 times higher than in pregnant women (511.2 vs 95.8 incidents per 100,000 women, respectively).
The incidence of DVT was 3 times higher than that of pulmonary embolism (151.8 vs 47.9 incidents, respectively, per 100,000 women). Pulmonary embolism was relatively less common during pregnancy than in the postpartum period (10.6 vs 159.7 incidents, respectively, per 100,000 women, respectively). [24] A national review of severe obstetric complications from 1998-2005 found a significant increase in the rate of pulmonary embolism associated with the increasing rate of cesarean delivery. [34]
Pulmonary embolism and postoperative mortality
Pulmonary embolism may account for 15% of all postoperative deaths. Leg amputations and hip, pelvic, and spinal surgery are associated with the highest risk.
Prognosis
The prognosis of patients with PE depends on two factors: the underlying disease state and appropriate diagnosis and treatment. Approximately 10% of patients who develop pulmonary embolism die within the first hour, and 30% die subsequently from recurrent embolism. Mortality for acute pulmonary embolism can be broken down into two categories: massive pulmonary embolism and nonmassive pulmonary embolism.
Anticoagulant treatment decreases mortality to less than 5%. At 5 days of anticoagulant therapy, 36% of lung scan defects are resolved; at 2 weeks, 52% are resolved; and at 3 months, 73% are resolved. Most patients treated with anticoagulants do not develop long-term sequelae upon follow-up evaluation. The mortality in patients with undiagnosed pulmonary embolism is 30%.
In the PIOPED study, the 1-year mortality rate was 24%. [35] The deaths occurred due to cardiac disease, recurrent pulmonary embolism, infection, and cancer.
The risk of recurrent pulmonary embolism is due to the recurrence of proximal venous thrombosis; approximately 17% of patients with recurrent pulmonary embolism were found to have proximal DVT. In a small proportion of patients, pulmonary embolism does not resolve; hence, chronic thromboembolic pulmonary arterial hypertension results.
Elevated plasma levels of natriuretic peptides (brain natriuretic peptide and N -terminal pro-brain natriuretic peptide) have been associated with higher mortality in patients with pulmonary embolism. [36] In one study, levels of N -terminal pro-brain natriuretic peptide greater than 500 ng/L were independently associated with central pulmonary embolism and were a possible predictor of death from pulmonary embolism. [37]
In a study of 270 adult patients with symptomatic pulmonary embolism that was objectively confirmed, researchers found that elevated plasma lactate levels (≥2 mmol/L) were associated with an increased risk of mortality and other adverse outcomes, independent of shock, hypotension, right-sided ventricular dysfunction, or injury markers. [38]
Massive pulmonary embolism
As a cause of sudden death, massive pulmonary embolism is second only to sudden cardiac death. Massive pulmonary embolism is defined as presenting with a systolic arterial pressure less than 90 mm Hg. The mortality for patients with massive pulmonary embolism is between 30% and 60%, depending on the study cited. [25, 39, 40] Autopsy studies of patients who died unexpectedly in a hospital setting have shown approximately 80% of these patients died from massive pulmonary embolism.
The majority of deaths from massive pulmonary embolism occur in the first 1-2 hours of care, so it is important for the initial treating physician to have a systemized, aggressive evaluation and treatment plan for patients presenting with pulmonary embolism.
Nonmassive pulmonary embolism
Nonmassive pulmonary embolism is defined as having a systolic arterial pressure greater than or equal to 90 mm Hg. This is the more common presentation for pulmonary embolism and accounts for 95.5-96% of the patients. [39, 41]
Hemodynamically stabile pulmonary embolism has a much lower mortality rate because of treatment with anticoagulant therapy. In nonmassive pulmonary embolism, the death rate is less than 5% in the first 3-6 months of anticoagulant treatment. The rate of recurrent thromboembolism is less than 5% during this time. However, recurrent thromboembolism reaches 30% after 10 years. [30]
Patient Education
The importance of adherence to the treatment regimen should be repeatedly stressed. The patient should be instructed regarding what to do in the event of any bleeding complications. Because most patients are administered warfarin or low molecular weight heparin upon discharge from the hospital, they must be advised regarding potential interactions between these agents and other medications.
For patient education resources, see the patient education articles Pulmonary Embolism and Blood Clot in the Legs.
-
A large pulmonary artery thrombus in a hospitalized patient who died suddenly.
-
Pulmonary embolism was identified as the cause of death in a patient who developed shortness of breath while hospitalized for hip joint surgery. This is a close-up view.
-
Lung infarction secondary to pulmonary embolism occurs rarely.
-
Posteroanterior and lateral chest radiograph findings are normal, which is the usual finding in patients with pulmonary embolism.
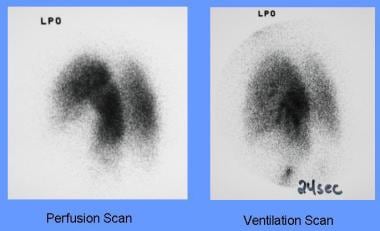
-
High-probability perfusion lung scan shows segmental perfusion defects in the right upper lobe and subsegmental perfusion defects in right lower lobe, left upper lobe, and left lower lobe.
-
A normal ventilation scan will make the noted defects in the previous image a mismatch and, hence, a high-probability ventilation-perfusion scan.
-
Anterior views of perfusion and ventilation scans are shown here. A perfusion defect is present in the left lower lobe, but perfusion to this lobe is intact, making this a high-probability scan.
-
A segmental ventilation perfusion mismatch is evident in a left anterior oblique projection.
-
A pulmonary angiogram shows the abrupt termination of the ascending branch of the right upper-lobe artery, confirming the diagnosis of pulmonary embolism.
-
A chest radiograph with normal findings in a 64-year-old woman who presented with worsening breathlessness.
-
This perfusion scan shows bilateral perfusion defects. The ventilation scan findings were normal; therefore, these are mismatches, and this is a high-probability scan.
-
This ultrasonogram shows a thrombus in the distal superficial saphenous vein, which is under the artery.
-
A posteroanterior chest radiograph showing a peripheral wedge-shaped infiltrate caused by pulmonary infarction secondary to pulmonary embolism. Hampton hump is a rare and nonspecific finding. Courtesy of Justin Wong, MD.
-
Computed tomography angiogram in a 53-year-old man with acute pulmonary embolism. This image shows an intraluminal filling defect that occludes the anterior basal segmental artery of the right lower lobe. Also present is an infarction of the corresponding lung, which is indicated by a triangular, pleura-based consolidation (Hampton hump).
-
Computed tomography angiography in a young man who experienced acute chest pain and shortness of breath after a transcontinental flight. This image demonstrates a clot in the anterior segmental artery in the left upper lung (LA2) and a clot in the anterior segmental artery in the right upper lung (RA2).
-
Computed tomography angiogram in a 55-year-old man with possible pulmonary embolism. This image was obtained at the level of the lower lobes and shows perivascular segmental enlarged lymph nodes as well as prominent extraluminal soft tissue interposed between the artery and the bronchus.
-
Computed tomography venograms in a 65-year-old man with possible pulmonary embolism. This image shows acute deep venous thrombosis with intraluminal filling defects in the bilateral superficial femoral veins.
-
The pathophysiology of pulmonary embolism. Although pulmonary embolism can arise from anywhere in the body, most commonly it arises from the calf veins. The venous thrombi predominately originate in venous valve pockets (inset) and at other sites of presumed venous stasis. To reach the lungs, thromboemboli travel through the right side of the heart. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.
-
A spiral CT scan shows thrombus in bilateral main pulmonary arteries.
-
CT scan of the same chest depicted in Image 18. Courtesy of Justin Wong, MD.
-
Longitudinal ultrasound image of partially recanalized thrombus in the femoral vein at mid thigh.
-
Sequential images demonstrate treatment of iliofemoral deep venous thrombosis due to May-Thurner (Cockett) syndrome. Far left, view of the entire pelvis demonstrates iliac occlusion. Middle left, after 12 hours of catheter-directed thrombolysis, an obstruction at the left common iliac vein is evident. Middle right, after 24 hours of thrombolysis, a bandlike obstruction is seen; this is the impression made by the overlying right common iliac artery. Far left, after stent placement, image shows wide patency and rapid flow through the previously obstructed region. Note that the patient is in the prone position in all views. (Right and left are reversed.)
-
Lower-extremity venogram shows outlining of an acute deep venous thrombosis in the popliteal vein with contrast enhancement.
-
Lower-extremity venogram shows a nonocclusive chronic thrombus. The superficial femoral vein (lateral vein) has the appearance of 2 parallel veins, when in fact, it is 1 lumen containing a chronic linear thrombus. Although the chronic clot is not obstructive after it recanalizes, it effectively causes the venous valves to adhere in an open position, predisposing the patient to reflux in the involved segment.
-
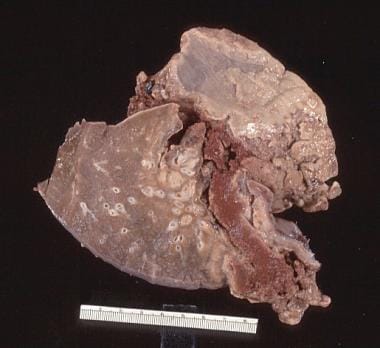
Pulmonary embolus.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Approach Considerations
- Clinical Scoring Systems
- D-Dimer Follow-Up on Low-to-Moderate Pretest Probability
- Ischemia-Modified Albumin levels
- White Blood Cell Count
- Arterial Blood Gases
- Troponin levels
- Brain Natriuretic Peptide
- Venography
- Angiography
- Computed Tomography Scanning
- Chest Radiography
- Ventilation-Perfusion Scanning
- Electrocardiography
- Magnetic Resonance Imaging
- Echocardiography
- Duplex Ultrasonography
- Show All
- Treatment
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- References