Practice Essentials
A pleural effusion is an abnormal collection of fluid in the pleural space resulting from excess fluid production or decreased absorption. [1] It is the most common manifestation of pleural disease. The pleural space is bordered by the parietal and visceral pleurae. The parietal pleura covers the inner surface of the thoracic cavity, including the mediastinum, diaphragm, and ribs. The visceral pleura envelops all lung surfaces, including the interlobar fissures. The right and left pleural spaces are separated by the mediastinum. [2, 3, 4]
The pleural space plays an important role in respiration by coupling the movement of the chest wall with that of the lungs in two ways. First, a relative vacuum in the space keeps the visceral and parietal pleurae in close proximity. Second, the small volume of pleural fluid, which has been calculated at 0.13 mL/kg of body weight under normal circumstances, serves as a lubricant to facilitate movement of the pleural surfaces against each other in the course of respirations. [5] This small volume of fluid is maintained through the balance of hydrostatic and oncotic pressure and lymphatic drainage, a disturbance of which may lead to pathology. [6] (See the images below.)
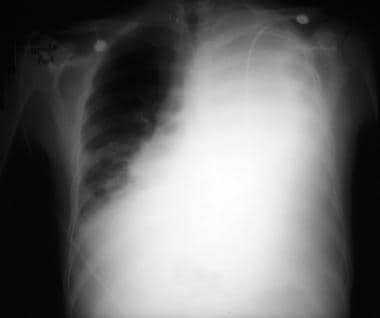 Emergent Management of Pleural Effusion. Anteroposterior upright chest radiograph shows a massive left-sided pleural effusion with contralateral mediastinal shift. Image courtesy of Allen R. Thomas, MD.
Emergent Management of Pleural Effusion. Anteroposterior upright chest radiograph shows a massive left-sided pleural effusion with contralateral mediastinal shift. Image courtesy of Allen R. Thomas, MD.
 Emergent Management of Pleural Effusion. Left lateral decubitus chest radiograph shows fluid layering on the left side, which is not a loculated effusion. Image courtesy of Allen R. Thomas, MD.
Emergent Management of Pleural Effusion. Left lateral decubitus chest radiograph shows fluid layering on the left side, which is not a loculated effusion. Image courtesy of Allen R. Thomas, MD.
Most commonly, a pleural effusion is an incidental finding in a stable patient. Prehospital interventions are generally limited to patients who are in respiratory distress or are hemodynamically unstable.
As with any other life-threatening condition, initial management is directed at ensuring adequate oxygenation and ventilation. Oxygen should be administered to all unstable patients. After airway stabilization, the patient's circulatory status should be assessed and supported as indicated.
After the initial stabilization of the patient, clinical suspicion for pleural effusion should be confirmed with appropriate radiographic evaluation. Chest radiography is the primary diagnostic tool. Emergency physicians may rapidly perform ultrasonography of the chest to evaluate patients with suspected pleural effusion. A spiral chest CT scan should be obtained for most patients with pleural effusion when the condition’s etiology cannot be readily determined or when complicated pleural effusion (eg, empyema, malignancy) is suspected. [7, 8, 9, 10, 11, 12]
Diagnostic procedures include percutaneous pleural biopsy, open pleural biopsy, bronchoscopy, thoracoscopy, and thoracentesis.
Laboratory evaluation of patients with a pleural effusion is directed at first determining if the effusion is an exudate or a transudate. The distinction between transudate and exudate is generally made by measurement of serum and pleural fluid lactate dehydrogenase (LDH) and protein concentrations. [5, 13, 14, 15, 16]
Strict precautions are required in the handling of needles and bodily fluids, including pleural fluid. Reports exist of human immunodeficiency virus (HIV) transmission from needles contaminated with pleural fluid.
Differential Diagnoses
Massive Effusions
A massive effusion is often attributable to an underlying malignancy. Other conditions that must be considered include the following:
-
Congestive heart failure
-
Tuberculosis (TB)
-
Cirrhosis with ascites
-
Transdiaphragmatic rupture of a liver abscess into the pleural space (>90% right-sided)
-
Paragonimiasis (usually unilateral)
-
Peritoneal dialysis (90% right sided)
-
Cryptococcosis
-
Pancreatic pseudocyst
-
Chronic pancreatitis
-
Meigs syndrome
-
Uremic pleuritis
-
Yellow nail syndrome
-
Effects of medications
-
Porous diaphragm syndrome
Massive effusions usually have an accompanying mediastinal shift to the contralateral side. In the absence of mediastinal shift, the differential diagnosis is narrowed to carcinoma of the ipsilateral mainstem bronchus with or without ipsilateral lung atelectasis, fixed mediastinum caused by fibrosis or tumor infiltration of the mediastinal lymph nodes, tumor infiltration of the ipsilateral lung, malignant mesothelioma, or complete atelectasis of the ipsilateral lung.
Bilateral Effusions
Bilateral effusions with an enlarged cardiac silhouette are most likely due to congestive heart failure. In the absence of cardiomegaly, malignancy (either lymphoma or carcinoma, with the exception of breast and lung cancer) is the most common cause. Other possible etiologies include the following:
-
Lupus pleuritis
-
Rheumatoid pleurisy
-
Nephrotic syndrome
-
Cirrhosis with ascites
-
Pulmonary embolism
-
TB
-
Esophageal rupture
-
Benign asbestos pleural effusion
-
Meigs syndrome
-
Uremic pleuritis
-
Yellow nail syndrome
-
Medication-associated effusion
Right-sided Effusions
The location of the pleural effusion can help in the differential diagnosis. Isolated, right-sided pleural effusions commonly occur with the following:
-
Cirrhosis
-
Peritoneal dialysis
-
Subphrenic or intrahepatic abscess
-
Amebic liver abscess
-
Echinococcal infection
-
Liver transplantation
-
Meigs syndrome
-
Catamenial hemothorax (thoracic endometriosis)
Left-sided Effusions
Isolated left-sided effusions occur with the following:
-
Esophageal rupture
-
Pancreatic disease
-
Subphrenic or splenic abscess
-
Splenic infarction
-
Diaphragmatic hernia
-
Pericardial disease
-
Following coronary artery bypass graft surgery
Air-fluid Levels
When an air-fluid level is present in the pleural space, the following must be considered:
-
Bronchopleural fistula
-
Pneumothorax
-
Trauma
-
Presence of gas-forming organisms
-
Diaphragmatic hernia
-
Fluid-filled bullae or lung cysts
-
Rupture of the esophagus into the pleural space
Diaphragmatic hernias can be excluded or confirmed with the administration of GI contrast material.
Loculated Effusions
Conditions associated with intense inflammation of the pleura can produce pleural adhesions, resulting in loculated effusions. When a loculated effusion is present, the following must be considered:
-
Empyema
-
Tuberculosis
-
Hemothorax
-
Chylothorax
Differentiating Exudate From Transudate
Laboratory evaluation of patients with a pleural effusion is directed at first determining if the effusion is an exudate or a transudate. An exudate tends to suggest a local process adjacent to or involving the pleura, whereas a transudate suggests a systemic process. As a consequence, with few exceptions, patients who present with a new pleural effusion should undergo a diagnostic thoracentesis. Subsequent testing is aimed at further identifying the underlying etiology or grading the severity of disease. Depending on the clinical setting, this evaluation may be completed in the emergency department (ED) or initiated in the ED and completed in an inpatient service. [13, 17]
The distinction between transudate and exudate is generally made by measurement of serum and pleural fluid lactate dehydrogenase (LDH) and protein concentrations.
Exudates have a higher protein concentration because of increased capillary permeability or decreased lymphatic drainage. As a result, the pleural effusion generally is an exudate if the pleural fluid-to-serum total protein ratio is greater than 0.50, if the pleural fluid LDH is greater than 0.67 of the upper limits of normal serum LDH, or if the pleural fluid-to-serum LDH ratio greater than 0.6. These criteria may misclassify up to 20% of transudates as exudates, especially if the patient was taking diuretics prior to the thoracentesis. [2]
A meta-analysis suggested that an exudate is more likely if the pleural fluid cholesterol is greater than 55 mg/dL, if the LDH is greater than 200 U/L, or if the ratio of pleural fluid cholesterol to serum cholesterol exceeds 0.3. An exudate was found to be less likely when all Light's criteria were absent (ie, a ratio of pleural fluid protein to serum protein greater than 0.5, a ratio of pleural fluid LDH to serum LDH greater than 0.6, or pleural fluid LDH greater than 0.67 of the upper limit of normal for serum LDH). [13]
Further Exudate Analysis
Exudative effusions require further laboratory investigation. The pleural fluid should be analyzed for the following:
-
Cell count with differential: A nucleated cell count less than 1000/μL suggests a transudate, whereas a count greater than 1000/μL suggests an exudate.
-
Glucose level: A level less than 60 mg/dL suggests complicated parapneumonic effusion or an effusion due to malignancy, tuberculosis, or rheumatoid disease.
-
Amylase level: Elevation suggests underlying pancreatitis or esophageal perforation. [2]
-
pH: Pleural fluid acidosis, defined as a pH less than 7.30, is seen in a limited number of conditions, including empyema; rheumatoid, tuberculous, or lupus pleuritis; malignancy; urinothorax; or esophageal rupture. [14]
-
Cytologic analysis: Especially if malignancy is suspected.
-
In the appropriate clinical setting, Gram staining, acid-fast bacilli staining, and culture and sensitivity for aerobic and anaerobic organisms and fungi.
Additional studies should be requested on the basis of the gross appearance of the pleural fluid or when a specific condition is suspected.
Procalcitonin and C-reactive protein are not routinely used in the investigation of pleural effusions; however, their utility is being studied:
-
C-reactive protein (CRP) - A CRP level >1.38 mg/dL has been found to be suggestive of parapneumonic effusion, while a level < 0.64 mg/dL may suggest pleural effusion due to heart failure. [16]
Chest Radiography
After the initial stabilization of the patient, clinical suspicion for pleural effusion should be confirmed with appropriate radiographic evaluation. The most frequently ordered studies are chest radiography, ultrasonography, and computed tomography (CT) scanning. Chest radiography has historically been the primary diagnostic tool because of its availability and low cost. It may also confirm the presence of effusion and suggest the underlying etiology. [3, 19]
Pleural fluid typically collects in the most dependent portion of the pleural space on an upright chest radiograph, primarily the posterior costophrenic recess, followed by the lateral recess. As little as 50 mL of fluid will cause blunting of the posterior costophrenic recess on a lateral upright film, whereas 200 mL is required to cause blunting of the lateral recess on a posteroanterior (PA) film. (See the images below.) However, as much as 500 mL of fluid in the pleural space may appear normal on chest radiograph. [10] Consequently, chest radiographs may miss a significant number of pleural effusions, including more than 10% of parapneumonic effusions large enough to warrant drainage. [20] Small to moderate pleural effusions are also frequently misdiagnosed as parenchymal opacities or not seen at all on portable anteriorposterior (AP) films. [21]
Loculated pleural effusions (eg, empyema, hemothorax) are not free moving because of adhesions between the parietal and visceral pleura and will often appear fixed in space with convex, sharply defined medial borders. [10] These effusions can be further characterized by ultrasonography and CT imaging (see images below).
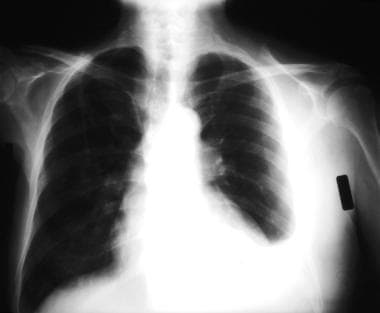 Emergent Management of Pleural Effusion. Posteroanterior upright chest radiograph shows isolated left sided pleural effusion and loss of left lateral costophrenic angle. Image courtesy of Allen R. Thomas, MD.
Emergent Management of Pleural Effusion. Posteroanterior upright chest radiograph shows isolated left sided pleural effusion and loss of left lateral costophrenic angle. Image courtesy of Allen R. Thomas, MD.
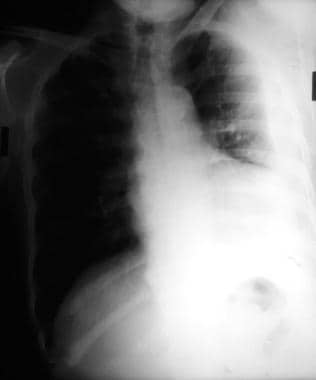
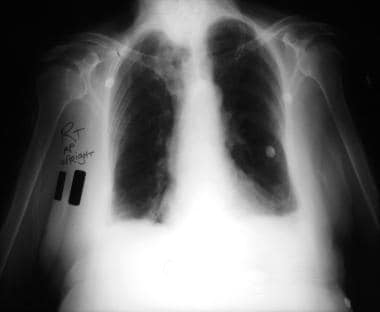 Emergent Management of Pleural Effusion. Anteroposterior upright chest radiograph shows bilateral pleural effusions and loss of bilateral costophrenic angles (meniscus sign). Image courtesy of Allen R. Thomas, MD.
Emergent Management of Pleural Effusion. Anteroposterior upright chest radiograph shows bilateral pleural effusions and loss of bilateral costophrenic angles (meniscus sign). Image courtesy of Allen R. Thomas, MD.
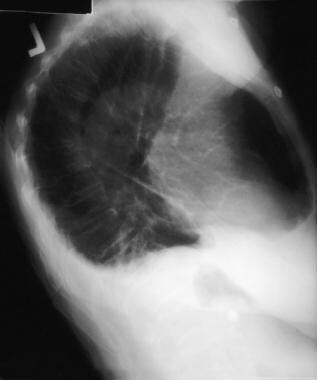 Emergent Management of Pleural Effusion. Chest radiograph, lateral view shows loss of bilateral posterior costophrenic angles. Image courtesy of Allen R. Thomas, MD.
Emergent Management of Pleural Effusion. Chest radiograph, lateral view shows loss of bilateral posterior costophrenic angles. Image courtesy of Allen R. Thomas, MD.
Chest radiography may yield other findings of value in determining the underlying disease process, such as pleural thickening or plaques, pulmonary parenchymal changes, or mediastinal enlargement. See the images below.
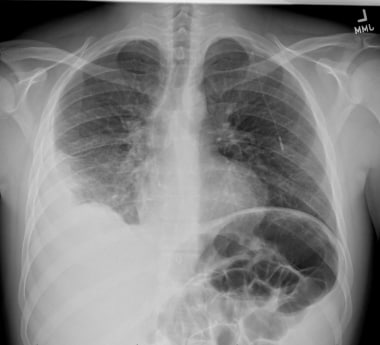 Emergent Management of Pleural Effusion. Posteroanterior upright chest radiograph shows a right basilar consolidation with a moderate right sided pleural effusion which was further characterized by ultrasound as containing loculations and subsequently by CT as an empyema.
Emergent Management of Pleural Effusion. Posteroanterior upright chest radiograph shows a right basilar consolidation with a moderate right sided pleural effusion which was further characterized by ultrasound as containing loculations and subsequently by CT as an empyema.
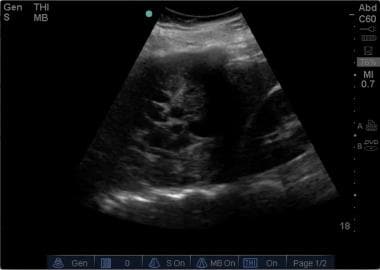
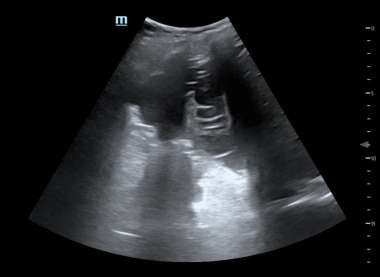 Emergent Management of Pleural Effusion. Right lateral lung ultrasound shows a loculated pleural effusion.
Emergent Management of Pleural Effusion. Right lateral lung ultrasound shows a loculated pleural effusion.
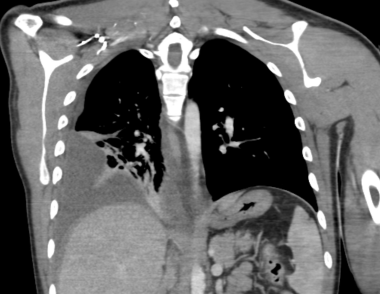 Emergent Management of Pleural Effusion. CT chest with IV contrast shows a loculated, moderate to large, right-sided pleural effusion with visceral pleural thickening and enhancement consistent with empyema.
Emergent Management of Pleural Effusion. CT chest with IV contrast shows a loculated, moderate to large, right-sided pleural effusion with visceral pleural thickening and enhancement consistent with empyema.
A lateral decubitus chest radiograph may detect as little as 5 mL of fluid. [7] Additional findings suggestive of pleural effusion include homogeneous opacification or diffuse haziness with a ground-glass appearance, visibility of pulmonary vessels through the haziness, and an absence of air bronchograms.
Ultrasonography
Mastery of bedside chest ultrasonography is becoming a requisite skill in many training programs, including critical care and emergency medicine. It has proven to be a valuable tool in rapidly diagnosing the etiology of undifferentiated dyspnea. [8, 22]
Emergency physicians may rapidly perform ultrasonography of the chest to evaluate patients with suspected pleural effusion. [9] It is more sensitive than chest radiography and physical exam in the diagnosis of pleural effusion. Furthermore, ultrasonography has the additional benefit of being readily available in most emergency departments, does not expose patients to radiation, and can be repeated for serial examinations. [11]
Ultrasonography detects as little as 5-50 mL of pleural fluid and has 100% sensitivity for effusions greater than 100 mL. It can identify loculated fluid collections, and it may detect pleura- or chest wall-based tumors. The appearance of the pleural effusion on ultrasonography may be suggestive of etiology: anechoic fluid in simple pleural effusion suggests a transudate, while complex pleural effusions with varying echogenicities are more frequently exudative. [11]
Ultrasonography can also be used to guide needle insertion for thoracentesis or chest tube placement [4, 23] and may be associated with a decreased procedural discomfort. [24] Use of intraprocedural ultrasonography has also been shown to significantly decrease complication rates from thoracentesis. [12]
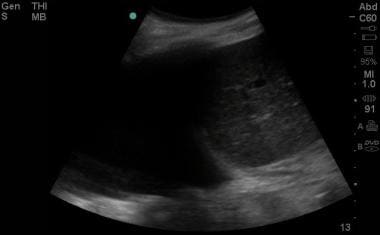 Emergent Management of Pleural Effusion. Ultrasound image of a large pleural effusion. Image courtesy of Michael A. Secko, MD.
Emergent Management of Pleural Effusion. Ultrasound image of a large pleural effusion. Image courtesy of Michael A. Secko, MD.
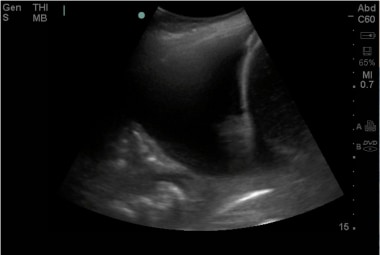 Emergent Management of Pleural Effusion. Ultrasound image of a large pleural effusion with associated pneumonia and atelectasis. Image courtesy of Michael A. Secko, MD.
Emergent Management of Pleural Effusion. Ultrasound image of a large pleural effusion with associated pneumonia and atelectasis. Image courtesy of Michael A. Secko, MD.
CT Scanning, MRI, and Echocardiography
CT Scanning
A spiral chest CT scan should be obtained for most patients with pleural effusion when the condition’s etiology cannot be readily determined or when complicated pleural effusion (eg, empyema, malignancy) is suspected. Scanning permits imaging of the entire pleural space, pulmonary parenchyma and vasculature, mediastinum, and pericardium. Abdominal slices may also be obtained if an intra-abdominal etiology for the effusion is suspected.
Chest CT scanning can help in distinguishing benign from malignant pleural involvement. One or more of the following suggests malignancy: circumferential pleural thickening, nodular pleural thickening, parietal pleural thickening (>1 cm), and mediastinal pleural involvement.
Contrast-enhanced “pleural phase” chest CT scans are able to distinguish between small effusions and pleural thickening. Contrast-enhanced chest CT scans are also able to readily distinguish between empyema, parapneumonic effusion, and pulmonary abscess. It is also useful in the evaluation of empyema refractory to treatment with antibiotics and chest tube drainage. [10]
MRI
Magnetic resonance imaging (MRI) may be used in evaluating patients with pleural effusion. Evaluation with MRI is limited, however, by motion artifact caused by breathing and cardiac activity. While it is not routinely utilized as part of an ED workup, it may be useful in the evaluation of the very young, of pregnant patients, or of those in whom contrast dye is contraindicated. [10] MRI may be helpful in distinguishing benign from malignant pleural disease. In addition, it complements CT scanning in evaluating patients with mesothelioma. [25]
Echocardiography
Echocardiography may be performed to further assess patients with pleural effusion related to congestive heart failure or if pericardial effusion is suspected.
Other Imaging Studies
Additional diagnostic testing is guided by the suspected etiology of the effusion and the patient's clinical status.
Esophageal perforation is a medical emergency that requires rapid diagnosis and treatment. When this condition is suspected, contrast-enhanced studies of the esophagus should be performed (and surgical consultation should be obtained early in the patient's evaluation).
In many EDs, helical chest CT scanning has replaced ventilation-perfusion (V-Q) scanning in the evaluation of patients with suspected pulmonary embolism. However, V-Q scans still have a role in some settings, including when a patient is not a candidate for a CT scan.
Thoracentesis
After the presence of a pleural effusion is established, diagnostic or therapeutic procedures may be considered (including thoracentesis, chest tube placement, percutaneous pleural biopsy, bronchoscopy, thoracoscopy, and open pleural biopsy). Thoracentesis and chest tube placement usually suffice for evaluation or treatment in the ED.
Thoracentesis, which involves the removal of 50-100 mL for laboratory analysis, is the first-line invasive diagnostic procedure and can be safely performed in most patients, including those undergoing mechanical ventilation. Thoracentesis can also be used as a therapeutic modality, in which greater volumes of pleural fluid are removed to alleviate dyspnea caused by large pleural effusion. Most patients with a new pleural effusion should undergo or be referred for a diagnostic thoracentesis. However, clinically stable, afebrile patients with clear diagnoses (eg, congestive heart failure [CHF], recent thoracic surgery, or known renal or hepatic disease) may not require thoracentesis. In these cases, treatment of the underlying etiology may be appropriate. If the effusion does not improve or if the patient becomes more symptomatic, thoracentesis may be considered at that time.
The most common complications of thoracentesis are pneumothorax, cough, and infection. Less common complications include hemothorax, liver or splenic puncture, intra-abdominal hemorrhage, unilateral pulmonary edema, air embolism, and shearing off of a catheter fragment within the pleural space. These risks are reduced with the intra-procedural use of ultrasound guidance. [12]
There are no absolute contraindications to thoracentesis. The use of ultrasound guidance has increased the safety of thoracentesis in patients with abnormal coagulation profiles. [26] It has significantly reduced the risk of procedural complications and increased procedural success rates. [12]
A chest radiograph is frequently obtained following thoracentesis to assess for pneumothorax. However, this may not be necessary when the procedure has involved only a single pass; when the patient has no risk of pleural adhesions; and when the patient remains asymptomatic. Obtain a post-procedural radiograph in newly symptomatic patients; after multiple passes, aspirated air, or severe underlying parenchymal lung disease; or in patients on mechanical ventilation. [26]
For massive effusions with a midline mediastinum or ipsilateral mediastinal shift, consultation with a pulmonologist is indicated before any intervention. In such patients, bronchoscopy, rather than thoracentesis, is generally the initial diagnostic procedure of choice.
Large-volume thoracentesis, usually defined as >1500 mL, may be associated with reexpansion pulmonary edema. [26, 17]
Emergency Department Care
On the basis of presentation in the ED, patients with pleural effusions may be (1) stable and require hospital admission, (2) stable and not require hospital admission, or (3) unstable. Generally, any patient who requires thoracentesis in the ED should be admitted to the hospital.
Stable patients who do not require admission include those in whom the clinical circumstances clearly explain the effusion, prior investigations of the cause were performed, effusions are typical of their disease and are asymptomatic, and diagnostic or therapeutic thoracentesis is not required.
In such patients, thoracentesis is not indicated emergently and can be deferred. Therapy for the specific cause of the effusion, if indicated, should be initiated. If the patient does not improve after a few days, diagnostic thoracentesis should be performed. This assumes that the patient is reliable, has a stable social situation, and has a physician with whom to follow-up.
Stable patients requiring admission include those with no prior history of pleural effusion, patients with parapneumonic effusions who do not appear to be septic, and patients with a prior history of pleural effusion whose condition has deteriorated. Although these patients are not in acute respiratory distress, diagnostic thoracentesis is warranted. This need not be performed in the ED if it can be performed promptly by the accepting inpatient service. When the cause of the pleural effusion is obvious, appropriate medical therapy should be initiated in the ED.
For suspected parapneumonic effusions, appropriate antibiotics should be administered in the ED, including coverage for anaerobic organisms.
Simple parapneumonic effusions have the potential to become complicated effusions or empyemas. Antimicrobial therapy alone is not sufficient for complicated parapneumonic effusions or empyemas. In these cases, prompt tube thoracostomy and antibiotics are required.
Unstable patients include those in septic shock or respiratory distress or with hemodynamic compromise due to the effusion. The initial treatment focus should be on stabilization of the patient. Patients with dyspnea or severe respiratory distress should be placed on the gurney in an upright position, as this will increase tidal volume and decrease the work of breathing and may improve symptoms of congestive heart failure and/or pulmonary edema.
Life-threatening traumatic or medical conditions (eg, tension hydropneumothorax, massive effusion with contralateral mediastinal shift, pulmonary embolism, esophageal perforation, traumatic rupture of the thoracic duct, strangulated diaphragmatic hernia) must be ruled out. These patients require immediate diagnostic and therapeutic thoracentesis.
Tube thoracostomy
Tube thoracostomy is indicated for the initial management of empyema, diagnosed by the presence of pus on thoracentesis, a positive Gram stain, fluid glucose of less than 60 mg/dL, a pH of less than 7.20, or an elevated LDH level. [27] Tube thoracostomy should also be performed on patients with hemothorax or pneumothorax.
The chest tube tip should be directed posteroinferiorly to drain blood or pus and superiorly in the setting of pneumothorax. Other conditions, such as complicated parapneumonic effusion, chylothorax, or malignant pleural effusion, may require chest tube placement for definitive treatment. However, this decision should be made in collaboration with the admitting service.
Placement of a chest tube in the setting of a malignant tumor that obstructs a mainstem or lobar bronchus is contraindicated, because the obstruction prevents expansion of the lung underlying the effusion.
Inpatient Care
The patient's condition and the cause of effusion dictate whether admission to a regular floor or the intensive care unit (ICU) is required. Consultation with pulmonary specialists or surgeons may facilitate level-of-care issues.
Repeated pleural aspiration for symptomatic relief is appropriate for patients with malignant pleural effusion whose life expectancy is less than 1 month. [28] For other patients, treatment may include serial thoracenteses, placement of a small-caliber chest tube for continuous drainage, a tunneled pleural catheter, chemical pleurodesis, pleuroperitoneal shunt placement, intrapleural administration of talc during thoracoscopy, systemic chemotherapy, or mediastinal radiation. [29] Intrapleural fibrinolytic therapy may be considered for patients with loculated parapneumonic effusions. [30]
Additional Treatment Considerations
Depending on the patient's clinical condition and local consultation practices, the primary care provider, a pulmonologist, or, if indicated, a medical or surgical intensivist or general surgeon may be consulted.
Thoracoscopic pleural biopsy has been shown to have diagnostic benefit when thoracentesis findings are indeterminate. [31]
Follow-up with the patient's primary care physician or a pulmonary specialist within 2-3 days of treatment is advisable, especially if thoracentesis is deferred. If early follow-up seems unlikely, the patient should be given clear instructions to return to the ED in 2-3 days for reevaluation.
Outpatient medication therapy is directed at the underlying etiology of the effusion. A social services professional should be consulted when a patient cannot afford prescribed medications.
Transfer
If the usual criteria for stability are satisfied, patients may be transferred to another facility for definitive care. If thoracentesis is performed, a follow-up chest radiograph should be obtained to rule out pneumothorax before transferring the patient.
In the case of iatrogenic pneumothorax, a chest tube should be placed prior to transfer. Stable patients may be transferred by ground with proper personnel and chest tube in place.
Guidelines Summary
The American Thoracic Society, Society of Thoracic Surgeons, and Society of Thoracic Radiology (ATS/STS/STR) released guidelines on the management of malignant pleural effusions on October 1, 2018. [32, 33, 34] Key points of the Malignant Pleural Effusions Clinical Practice Guidelines include the following:
-
Use ultrasound to guide pleural interventions in known or suspected cases of malignant pleural effusion.
-
Therapeutic pleural interventions should not be used in asymptomatic patients with malignant pleural effusion.
-
Use either an indwelling pleural catheter or chemical pleurodesis to manage dyspnea in symptomatic patients with malignant pleural effusion and known or suspected expandable lung.
-
Perform large-volume thoracentesis to assess lung expansion in cases where it is unclear if symptoms are associated with effusion and/or to see if the lung is expandable.
-
Use either talc poudrage or talc slurry for patients undergoing talc pleurodesis.
-
Use an indwelling pleural catheter instead of chemical pleurodesis in patients with nonexpandable lung, failed pleurodesis, or loculated effusion.
-
Use antibiotics to treat infections associated with the indwelling pleural catheter with the catheter in place. Only remove the catheter if the infection persists.
-
Emergent Management of Pleural Effusion. Anteroposterior upright chest radiograph shows a massive left-sided pleural effusion with contralateral mediastinal shift. Image courtesy of Allen R. Thomas, MD.
-
Emergent Management of Pleural Effusion. Anteroposterior upright chest radiograph shows bilateral pleural effusions and loss of bilateral costophrenic angles (meniscus sign). Image courtesy of Allen R. Thomas, MD.
-
Emergent Management of Pleural Effusion. Chest radiograph, lateral view shows loss of bilateral posterior costophrenic angles. Image courtesy of Allen R. Thomas, MD.
-
Emergent Management of Pleural Effusion. Posteroanterior upright chest radiograph shows isolated left sided pleural effusion and loss of left lateral costophrenic angle. Image courtesy of Allen R. Thomas, MD.
-
Emergent Management of Pleural Effusion. Left lateral decubitus chest radiograph shows fluid layering on the left side, which is not a loculated effusion. Image courtesy of Allen R. Thomas, MD.
-
Emergent Management of Pleural Effusion. Ultrasound image of a large pleural effusion. Image courtesy of Michael A. Secko, MD.
-
Emergent Management of Pleural Effusion. Ultrasound image of a large pleural effusion with associated pneumonia and atelectasis. Image courtesy of Michael A. Secko, MD.
-
Emergent Management of Pleural Effusion. Ultrasound image of a loculated pleural effusion. Image courtesy of Michael A. Secko, MD.
-
Emergent Management of Pleural Effusion. Posteroanterior upright chest radiograph shows a right basilar consolidation with a moderate right sided pleural effusion which was further characterized by ultrasound as containing loculations and subsequently by CT as an empyema.
-
Emergent Management of Pleural Effusion. Right lateral lung ultrasound shows a loculated pleural effusion.
-
Emergent Management of Pleural Effusion. CT chest with IV contrast shows a loculated, moderate to large, right-sided pleural effusion with visceral pleural thickening and enhancement consistent with empyema.
Tables
What would you like to print?
- Practice Essentials
- DDx
- Differentiating Exudate From Transudate
- Further Exudate Analysis
- Chest Radiography
- Ultrasonography
- CT Scanning, MRI, and Echocardiography
- Other Imaging Studies
- Thoracentesis
- Emergency Department Care
- Inpatient Care
- Additional Treatment Considerations
- Guidelines Summary
- Show All
- Media Gallery
- References