Practice Essentials
Atrial tachycardia is a supraventricular tachycardia (SVT) that does not require the atrioventricular (AV) junction, accessory pathways, or ventricular tissue for its initiation and maintenance. In addition to individuals with heart diseases, including congenital heart disease, atrial tachycardia may also occur in persons with structurally normal hearts. See the image below.
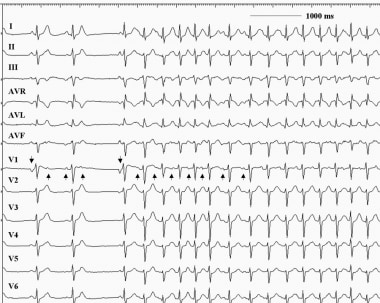 Atrial tachycardia. This 12-lead electrocardiogram demonstrates an atrial tachycardia at a rate of approximately 150 beats per minute. Note that the negative P waves in leads III and aVF (upright arrows) are different from the sinus beats (downward arrows). The RP interval exceeds the PR interval during the tachycardia. Note also that the tachycardia persists despite the atrioventricular block.
Atrial tachycardia. This 12-lead electrocardiogram demonstrates an atrial tachycardia at a rate of approximately 150 beats per minute. Note that the negative P waves in leads III and aVF (upright arrows) are different from the sinus beats (downward arrows). The RP interval exceeds the PR interval during the tachycardia. Note also that the tachycardia persists despite the atrioventricular block.
In clinical practice, three major types of atrial tachycardia are seen: focal atrial tachycardia, multifocal atrial tachycardia (MAT), and re-entrant atrial tachycardia. These arrhythmias have unique arrhythmic substrates and characteristics.
Focal atrial tachycardia arises from a localized atrial site and is characterized by regular, organized atrial activity with discrete P waves and, typically, an isoelectric segment between P waves. At times, irregularity is seen, especially at onset (“warm-up”) and termination (“warm-down”). Atrial mapping reveals a focal point of origin.
MAT is an irregular supraventricular tachycardia characterized by three distinct P-wave morphologies and/or patterns of atrial activation at different rates. The rhythm is always irregular.
Re-entrant atrial tachycardia is usually seen after cardiac surgery or catheter ablation with linear lesions that result in islets of scars. Re-entrant atrial tachycardias are usually incessant and may lead to cardiomyopathy.
In patients with structurally normal hearts, atrial tachycardia is associated with a low mortality rate. Patients with underlying structural heart disease, congenital heart disease, or lung disease are less likely to be able to tolerate this rhythm disturbance.
Signs and symptoms
Manifestations of atrial tachycardia include the following:
-
Rapid pulse rate: In most atrial tachycardias, the rapid pulse is regular; it may be irregular in rapid atrial tachycardias with variable AV conduction and in multifocal atrial tachycardia (MAT)
-
Episodic or paroxysmal occurrence
-
Sudden onset of palpitations
-
Continuous, sustained, or repetitive tachycardia: If atrial tachycardia is due to enhanced automaticity
-
"Warm-up" and "cool-off" phenomenon: Tachycardia gradually speeds up soon after onset (may be clinically inapparent); gradually slows down before termination
-
Dyspnea, dizziness, lightheadedness, fatigue, or chest pressure: In tachycardic episodes accompanied by palpitations
-
Syncope: With rapid rate and severe hypotension
-
Heart-failure symptoms and reduced effort tolerance: Early manifestations of tachycardia-induced cardiomyopathy in patients with frequent or incessant tachycardia
In patients with MAT, the history may disclose an underlying illness that is causing the tachycardia. Such illnesses include pulmonary, cardiac, metabolic, and endocrinopathic disorders. Chronic obstructive pulmonary disease (COPD) is the most common underlying disease process (60%) in MAT.
Reentrant atrial tachycardia is not uncommon in patients with a history of a surgically repaired atrial septal defect. The scar tissue in the atrium may give rise to the formation of a reentrant circuit.
On physical examination, the primary abnormal finding is a rapid pulse rate. The rate is usually regular, but it may be irregular in rapid atrial tachycardias with variable AV conduction and in MAT. Blood pressure may be low in patients presenting with fatigue, lightheadedness, or presyncope.
See Presentation for more detail.
Workup
Workup for atrial tachycardia can employ the following diagnostic tools:
-
12-lead electrocardiography (ECG) with rhythm strip: To help identify, locate, and differentiate atrial tachycardia
-
Modified ECG with the Lewis lead: The right- and left-arm electrodes are applied at the two sides of the sternum at the second and fourth intercostal spaces, respectively; this may be used to magnify P waves.
-
Esophageal recording of atrial activation: May be necessary to discern P waves, especially in the pediatric age group
-
Holter monitoring: To analyze the onset and termination of atrial tachycardia, identify the AV conduction block during the episode, and correlate the symptoms to atrial tachycardia
-
Endocardial mapping: To localize atrial tachycardia
The following laboratory studies may be indicated to exclude systemic causes of sinus tachycardia:
-
Serum chemistry: To exclude electrolyte disorders
-
Blood hemoglobin level and red blood cell (RBC) counts: To seek evidence of anemia
-
Arterial blood gas level: To define pulmonary status
-
Serum digoxin assay: When digitalis intoxication is suspected
The following imaging studies can be useful in the evaluation of patients with atrial tachycardia:
-
Chest radiography: To assess pulmonary etiology (eg, COPD) and delineate cardiac size and structures (eg, in patients with tachycardia-induced cardiomyopathy or complex congenital heart disease)
-
Computed tomography (CT) scanning: To assess the anatomy of cardiac structures, including the pulmonary veins especially, to provide images prior to ablative procedure, and to exclude pulmonary embolism
-
Echocardiography: To assess structural heart disease, left atrial size, pulmonary arterial pressure, left ventricular function, and pericardial pathology
See Workup for more detail.
Treatment
The primary treatment during an episode of atrial tachycardia is considered to be ventricular rate control using AV nodal blocking agents (eg, beta-blockers, calcium channel blockers). Antiarrhythmic drugs can prevent recurrences and may be required; a calcium channel blocker or beta-blocker also may be required in combination therapy. Specific antiarrhythmic therapies include the following:
-
Atrial tachycardia from triggered activity: Verapamil, beta-blockers, and adenosine
-
Atrial tachycardia from enhanced automaticity: Beta-blockers, but overall success rates are low
-
Refractory recurrent atrial tachycardia: Class Ic antiarrhythmic drugs
-
Maintenance of sinus rhythm: Class III antiarrhythmic drugs
Nonpharmacologic therapies for atrial tachycardia include the following:
-
Cardioversion: For patients in whom the rhythm is not well-tolerated hemodynamically and/or in whom rate-control drugs are ineffective or contraindicated
-
Surgical ablation: For patients with complex congenital heart disease
Multifocal atrial tachycardia
Treatment of MAT involves treatment and/or reversal of the precipitating cause. Therapy also may include the following:
-
Calcium channel blockers: Used as the first line of treatment
-
Magnesium sulfate
-
Beta-blockers
-
Antiarrhythmics
In very rare cases, when MAT is persistent and refractory, AV junctional ablation and permanent pacemaker implantation may be considered. Such treatment can provide symptomatic and hemodynamic improvement and prevent the development of tachycardia-mediated cardiomyopathy, although patients may become pacemaker dependent. [3] In general, short and asymptomatic runs of atrial tachycardia detected as an incidental finding on ambulatory ECG do not require treatment.
See Treatment and Medication for more detail.
Background
Atrial tachycardia is defined as a supraventricular tachycardia (SVT) that does not require the atrioventricular (AV) junction, accessory pathways, or ventricular tissue for its initiation and maintenance. Atrial tachycardia can be observed in persons with normal hearts and in those with structurally abnormal hearts, including those with congenital heart disease and particularly after surgery for repair or correction of congenital or valvular heart disease.
In adults, tachycardia is usually defined as a heart rate of more than 100 beats per minute (bpm). In children, the definition of tachycardia varies because the normal heart rate is age dependent, as follows [4, 5] :
-
Age 1-2 days: 123-159 bpm
-
Age 3-6 days: 129-166 bpm
-
Age 1-3 weeks: 107-182 bpm
-
Age 1-2 months: 121-179 bpm
-
Age 3-5 months: 106-186 bpm
-
Age 6-11 months: 109-169 bpm
-
Age 1-2 years: 89-151 bpm
-
Age 3-4 years: 73-137 bpm
-
Age 5-7 years: 65-133 bpm
-
Age 8-11 years: 62-130 bpm
-
Age 12-15 years: 60-119 bpm
As in most SVTs, the electrocardiogram (ECG) typically shows a narrow QRS complex tachycardia (unless bundle branch block aberration occurs). Heart rates are highly variable, with a range of 100-250 bpm. The atrial rhythm is usually regular. (See the image below.)
 Atrial tachycardia. This 12-lead electrocardiogram demonstrates an atrial tachycardia at a rate of approximately 150 beats per minute. Note that the negative P waves in leads III and aVF (upright arrows) are different from the sinus beats (downward arrows). The RP interval exceeds the PR interval during the tachycardia. Note also that the tachycardia persists despite the atrioventricular block.
Atrial tachycardia. This 12-lead electrocardiogram demonstrates an atrial tachycardia at a rate of approximately 150 beats per minute. Note that the negative P waves in leads III and aVF (upright arrows) are different from the sinus beats (downward arrows). The RP interval exceeds the PR interval during the tachycardia. Note also that the tachycardia persists despite the atrioventricular block.
The conducted ventricular rhythm is also usually regular. It may become irregular, however, especially at higher atrial rates, because of variable conduction through the AV node, thus producing conduction patterns such as 2:1, 4:1, a combination of those, or Wenckebach AV block.
The P wave morphology on the ECG may give clues to the site of origin and mechanism of the atrial tachycardia. In the case of a focal tachycardia, the P wave morphology and axis depend on the location in the atrium from which the tachycardia originates. In the case of macroreentrant circuits, the P wave morphology and axis depend on activation patterns (see Workup).
Multifocal atrial tachycardia (MAT) is an arrhythmia with an irregular atrial rate greater than 100 bpm. Atrial activity is well organized, with at least three morphologically distinct P waves, irregular P-P intervals, and an isoelectric baseline between the P waves. [6] Multifocal atrial tachycardia has previously been described by names such as chaotic atrial rhythm or tachycardia, chaotic atrial mechanism, and repetitive paroxysmal MAT. Go to Multifocal Atrial Tachycardia for more complete information on this topic.
Classification methods
A number of methods are used to classify atrial tachycardia. Classification in terms of origin can be based on endocardial activation mapping data, pathophysiologic mechanisms, and anatomy.
On the basis of endocardial activation, atrial tachycardia may be divided into the following two groups (see Presentation):
-
Focal atrial tachycardia: Arises from a localized area in the atria such as the crista terminalis, pulmonary veins, ostium of the coronary sinus, or intra-atrial septum.
-
Reentrant atrial tachycardias: Usually macroreentrant; reentrant atrial tachycardias most commonly occur in persons with either structural or complex heart disease, particularly after surgery involving atrial incisions or scarring
Other methods of classification are as follows:
-
Pathophysiologic mechanisms: Atrial tachycardia can be classified as the result of enhanced automaticity, triggered activity, or reentry (see Pathophysiology)
-
Anatomy: Classification of atrial tachycardia can be based on the location of the arrhythmogenic focus (see Anatomy)
Diagnosis and treatment
A 12-lead ECG with rhythm strip is an important tool to help identify, locate, and differentiate atrial tachycardia. Laboratory studies may be indicated to exclude systemic disorders that may be causing the tachycardia. Invasive electrophysiologic study (EPS) may be required. (See Workup.) The morphology of the P wave may provide valuable clues to the origin of the tachycardia, and this is the reason why a 12-lead ECG is of special value.
The primary treatment during an episode of atrial tachycardia is considered to be rate control using AV nodal blocking agents, such as beta-blockers or calcium channel blockers (see Treatment and Medication). Cardioversion should be considered for any patient in whom the rhythm is not tolerated well hemodynamically and/or in whom rate control drugs are ineffective or contraindicated.
Catheter ablation for atrial tachycardia has become a highly successful and effective treatment option for symptomatic patients whose condition is refractory to medical therapy or who do not desire long-term antiarrhythmic therapy. It can cure macroreentrant and focal forms of atrial tachycardia. (See Treatment.) [7, 8]
Anatomy
Atrial tachycardia can have a right or left atrial origin. Some atrial tachycardias actually originate outside the usual anatomic boundaries of the atria, in areas such as the superior vena cava, pulmonary veins, and vein of Marshall, where fingers of atrial myocardium extend into these locations. Rare locations, such as the noncoronary aortic cusp [1] and hepatic veins, have been described, as well. (See the video below.)
A number of aspects of the atrial anatomy can contribute to the substrate for arrhythmia. The orifices of the vena cava, pulmonary veins, coronary sinus, atrial septum, and mitral and tricuspid annuli are potential anatomic boundaries for reentrant circuits.
Anisotropic conduction in the atria due to complex fiber orientation may create the zone of slow conduction. Certain atrial tissues, such as the crista terminalis and pulmonary veins, are common sites for automaticity or triggered activity. Additionally, disease processes or age-related degeneration of the atria may give rise to the arrhythmogenic substrate.
Abnormalities that have been reported at the sites of atrial tachycardia origin include the following [9] :
-
Extensive myocardial fibrosis
-
Myocyte hypertrophy
-
Endocardial fibrosis
-
Mononuclear cell infiltration
-
Mesenchymal cell proliferation
-
Islets of fatty tissue
-
Thinning
-
Blebs
Pathophysiology
Several pathophysiologic mechanisms have been ascribed to atrial tachycardia. These mechanisms can be differentiated on the basis of the pattern of onset and termination and the response to drugs and atrial pacing.
Enhanced automaticity
Automatic atrial tachycardia arises due to enhanced tissue automaticity and is observed in patients with structurally normal hearts and in those with organic heart disease. The tachycardia typically exhibits a warm-up phenomenon, during which the atrial rate gradually accelerates after its initiation and slows prior to its termination.
Automatic atrial tachycardia is rarely initiated or terminated by a single atrial stimulation or rapid atrial pacing, but it may be transiently suppressed by overdrive pacing. It almost always requires isoproterenol infusion to facilitate induction and is predictably terminated by propranolol. [10] Carotid sinus massage and adenosine do not terminate the tachycardia even if they produce a transient AV nodal block. Electrical cardioversion is ineffective (being equivalent to attempting electrical cardioversion in a sinus tachycardia).
Triggered activity
Atrial tachycardia caused by triggered activity is due to delayed after-depolarizations, which are low-amplitude oscillations occurring at the end of the action potential. [11] These delayed after-depolarization–related oscillations are triggered by the preceding action potential and are the result of calcium ion influxes into the myocardium. If these oscillations are of sufficient amplitude to reach the threshold potential, depolarization occurs again and a spontaneous action potential is generated.
If single, this is recognized as an atrial ectopic beat (an extra or premature beat). If it recurs and spontaneous depolarization continues, a sustained tachycardia may result.
Most commonly, atrial tachycardia due to triggered activity occurs in patients with digitalis intoxication [2] or conditions associated with excess catecholamines. Characteristically, the arrhythmia can be initiated, accelerated, and terminated by rapid atrial pacing. It may be sensitive to physiologic maneuvers and drugs such as adenosine, verapamil, and beta-blockers, all of which can terminate the tachycardia.
Occasionally, this atrial tachycardia may arise from multiple sites in the atria, producing a multifocal or multiform atrial tachycardia. This may be recognized by varying P wave morphology and irregularity in the atrial rhythm.
Pulmonary vein tachycardias
Pulmonary vein tachycardias originate from the os of the pulmonary vein or even deeper localized atrial fibers. These strands of atrial tissue are generally believed to gain electrical independence, since they are partially isolated from the atrial myocardium. These tachycardias are typically very rapid (heart rate of 200-220 bpm or more)
Although pulmonary vein tachycardias frequently trigger episodes of atrial fibrillation, the associated atrial tachycardias may be the clinically dominant or exclusive manifestation. The latter typically involves only a single pulmonary vein as opposed to the multiple pulmonary vein involvement seen in atrial fibrillation.
Reentrant tachycardia
Intra-atrial reentry tachycardias may have either a macroreentrant or a microreentrant circuit. Macroreentry is the usual mechanism in atrial flutter and in scar- and incision-related (postsurgical) atrial tachycardia.
The more common and recognized form of atrial tachycardia, seen as a result of the advent of pulmonary vein isolation and linear ablation procedures, is left atrial tachycardia. In this situation, gaps in the ablation lines allow for slow conduction, providing the requisite anatomic substrate for reentry. These tachycardias may be self-limiting but if they persist, mapping and a repeat ablative procedure should be considered.
Microreentry can arise in a small focal area, such as in sinus node reentrant tachycardia. Typically, episodes of reentrant atrial tachycardia arise suddenly, terminate suddenly, and are paroxysmal. Carotid sinus massage and adenosine are ineffective in terminating macroreentrant tachycardias, even if they produce a transient AV nodal block. During electrophysiologic study, it can be induced and terminated by programmed extrastimulation. As is typical of other reentry tachycardias, electrical cardioversion terminates this type of atrial tachycardia.
Classification of atrial tachycardia
Based on endocardial activation, atrial tachycardia may be divided into two groups: focal and reentrant. Focal atrial tachycardia arises from a localized area in the atria such as the crista terminalis, pulmonary veins, ostium of the coronary sinus, or intra-atrial septum. If it originates from the pulmonary veins, it may trigger atrial fibrillation and often forms a continuum of arrhythmias.
Reentrant (usually macroreentrant) atrial tachycardias most commonly occur in persons with structural heart disease or complex congenital heart disease, particularly after surgery involving incisions or scarring in the atria. Electrophysiologically, these atrial tachycardias are similar to atrial flutters, typical or atypical. Often, the distinction is semantic, typically based on arbitrary cutoffs of atrial rate.
Some tachycardias cannot be easily classified. Reentrant sinoatrial tachycardia (or sinus node reentry) is a subset of focal atrial tachycardia due to reentry arising within the sinus node situated at the superior aspect of the crista terminalis. The P wave morphology and atrial activation sequence are identical or very similar to those of sinus tachycardia.
Etiology
Atrial tachycardia can occur in individuals with structurally normal hearts or in patients with organic heart disease. When it arises in patients with congenital heart disease who have undergone corrective or palliative cardiac surgery, such as a Fontan procedure, an atrial tachycardia can have potentially life-threatening consequences. [12]
The atrial tachycardia that manifests in association with exercise, acute illness with excessive catecholamine release, alcohol ingestion, altered fluid states, hypoxia, metabolic disturbance, or drug use (eg, caffeine, albuterol, theophylline, cocaine) is associated with automaticity or triggered activity. Digitalis intoxication is an important cause of atrial tachycardia, with triggered activity being the underlying mechanism.
Reentrant atrial tachycardia tends to occur in patients with structural heart disease, including ischemic, congenital, postoperative, and valvular disorders. Iatrogenic atrial tachycardias have become more common and typically result from ablative procedures in the left atrium. Several typical origination sites for these tachycardias have been identified, including the mitral isthmus (between the left lower pulmonary vein and mitral annulus), the roof of the left atrium, and, for reentry, around the pulmonary veins.
The most common reason for postablation tachycardias is gaps in the ablation lines, allowing for slow conduction and initiation reentry circuits or circuits excluded by the set of ablation lines. Typically, these patients have undergone an atrial fibrillation ablation procedure. This is true for catheter ablation and surgical epicardial ablation. Similarly, patients with prior surgical procedures involving the left atrium may have surgical incision lines and, hence, the potential for macroreentrant circuits.
MAT is often related to underlying illnesses, frequently occurring in patients experiencing an exacerbation of chronic obstructive pulmonary disease (COPD), [3] a pulmonary thromboembolism, an exacerbation of heart failure, or severe illness, especially under critical care with inotropic infusion. MAT is often associated with hypoxia and sympathetic stimulation. Digitalis toxicity also may be present in persons with MAT, with triggered activity as the mechanism.
Other underlying conditions that are commonly associated with MAT are the following:
-
Valvular heart disease
-
Diabetes mellitus
-
Hypokalemia
-
Hypomagnesemia
-
Azotemia
-
Postoperative state
-
Sepsis
-
Methylxanthine toxicity
-
Myocardial infarction
-
Pneumonia
Unusual forms of atrial tachycardias can be seen in patients with an infiltrative process involving the pericardium and, by extension, the atrial wall.
A very unusual form of recipient-to-donor atrial tachycardia may be seen in patients who have undergone bi-atrial orthotropic cardiac transplantation. [13] In this situation, atrial arrhythmia originates in the recipient remnant of the heart or from reentry around the incision suture lines and break through to the donor heart. Because of cardiac denervation, the recipient patients usually do not develop symptoms of palpitations but rather develop tachycardia-induced cardiomyopathy in the donor heart. These atrial tachycardias are extremely difficult to treat with drugs, but they can be cured by catheter ablation. Sometimes, atrial fibrillation and atrial flutter in the recipient heart indicate rejection; hence, close vigilance is necessary.
Epidemiology
Atrial tachycardia is relatively rare, constituting 5-15% of all SVTs. Atrial tachycardia has no known racial or ethnic predilection and no known predilection for either sex. There may be some association with pregnancy.
Atrial tachycardia may occur at any age, although it is more common in children and adults with congenital heart disease. MAT is a relatively infrequent arrhythmia, with a prevalence rate of 0.05-0.32% in patients who are hospitalized. It is predominantly observed in males and in older patients—in particular, elderly patients with multiple medical problems. The average age of patients from 9 studies was 72 years.
Prognosis
In patients with structurally normal hearts, atrial tachycardia is associated with a low mortality rate. However, tachycardia-induced cardiomyopathies have developed in patients with persistent or frequent atrial tachycardia. Patients with underlying structural heart disease, congenital heart disease, or lung disease are less likely to be able to tolerate atrial tachycardia. Other morbidity is associated with lifestyle changes and associated symptoms.
A study by Chung et al indicated that in patients with acute ischemic stroke and nonsustained atrial tachycardia, an enlarged left atrium is a risk factor for stroke recurrence. The study involved 252 patients, who were followed up for a mean period of 35 months. [14]
Multifocal atrial tachycardia
MAT itself is seldom life threatening. The condition is transient and resolves when the underlying condition improves. The prognosis depends on the prognosis of any comorbid disease.
Many patients with MAT have significant comorbidities, especially COPD and respiratory failure, that often require treatment in an intensive care unit. Consequently, a high mortality rate (up to 45%) is associated with this arrhythmia, although the mortality is not a direct consequence of the rhythm abnormality.
Potential complications of MAT include development of tachycardia-induced cardiomyopathy if the arrhythmia is persistent. Other complications include the following:
-
Atrial thrombi with embolization and subsequent stroke
-
Myocardial infarction from incongruous myocardial supply and demand
-
Pulmonary emboli
Patient Education
For patient education information, see the Heart Health Center, as well as Supraventricular Tachycardia and Palpitations.
In the case of multifocal atrial tachycardia (MAT) related to medication, education regarding correct medication usage and the monitoring of such medications should be considered. In the case of a pulmonary source, education about prevention and recognition of developing pulmonary conditions may be helpful.
-
Atrial tachycardia. This 12-lead electrocardiogram demonstrates an atrial tachycardia at a rate of approximately 150 beats per minute. Note that the negative P waves in leads III and aVF (upright arrows) are different from the sinus beats (downward arrows). The RP interval exceeds the PR interval during the tachycardia. Note also that the tachycardia persists despite the atrioventricular block.
-
Atrial tachycardia. This propagation map of a right atrial tachycardia originating from the right atrial appendage was obtained with non-contact mapping using the EnSite mapping system.
-
Atrial tachycardia. Note that the atrial activities originate from the right atrium and persist despite the atrioventricular block. These features essentially exclude atrioventricular nodal reentry tachycardia and atrioventricular tachycardia via an accessory pathway. Note also that the change in the P-wave axis at the onset of tachycardia makes sinus tachycardia unlikely.
-
Atrial tachycardia. An anterior-posterior mapping projection is shown. This is an example of activation mapping using contact technique and the EnSite system. The atrial anatomy is partially reconstructed. Early activation points are marked with white/red color. The activation waveform spreads from the inferior/lateral aspect of the atrium through the entire chamber. White points indicate successful ablation sites that terminated the tachycardia. CS = shadow of the catheter inserted in the coronary sinus; TV = tricuspid valve.
-
Atrial tachycardia. These intracardiac tracings showing atrial tachycardia breaking with the application of radiofrequency energy. Before ablation, the local electrograms from the treatment site preceded the surface P wave by 51 ms, consistent with this site being the source of the tachycardia. Note that postablation electrograms on the ablation catheter are inscribed well past the onset of the sinus rhythm P wave. The first three tracings show surface electrocardiograms as labeled. Abl = ablation catheter (D-distal pair of electrodes); CS = respective pair of electrodes of the coronary sinus catheter; CS 1,2 = distal pair of electrodes; CS 7,8 = electrodes located at the os of the coronary sinus.
-
Atrial tachycardia. This image shows an example of rapid atrial tachycardia mimicking atrial flutter. A single radiofrequency application terminates the tachycardia. The first three tracings show surface electrocardiograms, as labeled. AblD and AblP = distal and proximal pair of electrodes of the mapping catheter, respectively; HBED and HBEP = distal and proximal pair of electrodes in the catheter located at His bundle, respectively; HRA = high right atrial catheter; MAP = unipolar electrograms from the tip of the mapping catheter; RVA = catheter located in right ventricular apex.
-
Atrial tachycardia. This electrocardiogram shows multifocal atrial tachycardia (MAT).
-
Atrial tachycardia. This electrocardiogram belongs to an asymptomatic 17-year-old male who was incidentally discovered to have Wolff-Parkinson-White (WPW) pattern. It shows sinus rhythm with evident preexcitation. To locate the accessory pathway (AP), the initial 40 milliseconds of the QRS (delta wave) are evaluated. Note that the delta wave is positive in lead I and aVL, negative in III and aVF, isoelectric in V1, and positive in the rest of the precordial leads. Therefore, this is likely a posteroseptal AP.
-
Atrial tachycardia. This is a 12-lead electrocardiogram from an asymptomatic 7-year-old boy with Wolff-Parkinson-White (WPW) pattern. Delta waves are positive in leads I and aVL; negative in II, III, and aVF; isoelectric in V1; and positive in the rest of the precordial leads. This again predicts a posteroseptal location for the accessory pathway (AP).







