Practice Essentials
Myocardial infarction (MI) (ie, heart attack) is the irreversible death (necrosis) of heart muscle secondary to prolonged lack of oxygen supply (ischemia). Approximately 1.5 million cases of MI occur annually in the United States. See the images below.
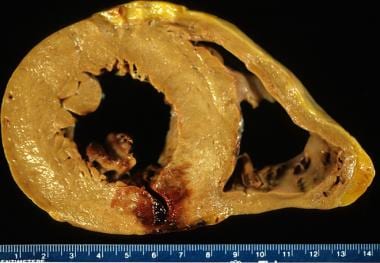 Acute myocardial infarction, reperfusion type. In this case, the infarct is diffusely hemorrhagic. There is a rupture track through the center of this posterior left ventricular transmural infarct. The mechanism of death was hemopericardium.
Acute myocardial infarction, reperfusion type. In this case, the infarct is diffusely hemorrhagic. There is a rupture track through the center of this posterior left ventricular transmural infarct. The mechanism of death was hemopericardium.
See Are You Missing Subtle MI Clues on ECGs? Test Your Skills, a Critical Images slideshow, to help identify a variety of electrocardiographic abnormalities.
Signs and symptoms
Patients with typical MI may have the following symptoms in the days or even weeks preceding the event (although typical STEMI may occur suddenly, without warning):
-
Fatigue
-
Chest discomfort
-
Malaise
Typical chest pain in acute MI has the following characteristics:
-
Intense and unremitting for 30-60 minutes
-
Substernal, and often radiates up to the neck, shoulder, and jaw, and down the left arm
-
Usually described as a substernal pressure sensation that also may be characterized as squeezing, aching, burning, or even sharp
-
In some patients, the symptom is epigastric, with a feeling of indigestion or of fullness and gas
The patient’s vital signs may demonstrate the following in MI:
-
The patient’s heart rate is often increased (tachycardic) secondary to a high sympathoadrenal discharge
-
The pulse may be irregular because of ventricular ectopy, an accelerated idioventricular rhythm, ventricular tachycardia, atrial fibrillation or flutter, or other supraventricular arrhythmias; bradyarrhythmias may be present
-
In general, the patient's blood pressure is initially elevated because of peripheral arterial vasoconstriction resulting from an adrenergic response to pain and ventricular dysfunction
-
However, with right ventricular MI or severe left ventricular dysfunction, hypotension and cardiogenic shock can be seen
-
The respiratory rate may be increased in response to pulmonary congestion or anxiety
-
Coughing, wheezing, and the production of frothy sputum may occur
See Clinical Presentation for more detail.
Diagnosis
Laboratory studies
Laboratory tests used in the diagnosis of MI include the following:
-
Cardiac biomarkers/enzymes: The American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) guidelines recommend that cardiac biomarkers should be measured at presentation in patients with suspected MI, and that the only biomarker that is recommended to be used for the diagnosis of acute MI at this time is cardiac troponin due to its superior sensitivity and accuracy. [1, 2, 3, 4]
-
Troponin levels: Troponin is a contractile protein that normally is not found in serum; it is released only when myocardial necrosis occurs
-
Complete blood cell count
-
Comprehensive metabolic panel
-
Lipid profile
Electrocardiography
The ECG is the most important tool in the initial evaluation and triage of patients in whom an acute coronary syndrome (ACS), such as MI, is suspected. It is confirmatory of the diagnosis in approximately 80% of cases.
Cardiac imaging
For individuals with highly probable or confirmed acute MI, coronary angiography can be used to definitively diagnose or rule out coronary artery disease.
See Workup for more detail.
Management
Prehospital care
For patients with chest pain, prehospital care includes the following:
-
Intravenous access, supplemental oxygen if SaO2 is less than 90%, pulse oximetry
-
Immediate administration of nonenteric-coated chewable aspirin
-
Nitroglycerin for active chest pain, given sublingually or by spray
-
Telemetry and prehospital ECG, if available
Emergency department and inpatient care
Initial stabilization of patients with suspected MI and ongoing acute chest pain should include administration of sublingual nitroglycerin if patients have no contraindications to it.
The American Heart Association (AHA) recommends the initiation of beta blockers to all patients with STEMI (unless beta blockers are contraindicated). [1, 2]
If STEMI is present and the patient is within 90 minutes of a PCI-capable facility, the patient should undergo emergent coronary angiography and primary PCI. If the patient is longer than 120 minutes from a PCI-capable facility, fibrinolysis should be considered. [2]
Although patients presenting without ST-segment elevation (non-STE-ACS) are not candidates for immediate administration of thrombolytic agents, they should receive anti-ischemic therapy and may be candidates for PCI urgently or during admission.
Coronary care units have reduced early mortality rates from acute MI by approximately 50% by providing immediate defibrillation and by facilitating the implementation of beneficial interventions. These interventions include the administration of intravenous (IV) medications and therapy designed to do the following:
-
Limit the extent of MI
-
Salvage jeopardized ischemic myocardium
-
Recanalize infarct-related arteries
See Treatment and Medication for more detail.
Background
Myocardial infarction (MI) usually results from an imbalance in oxygen supply and demand, which is most often caused by plaque rupture with thrombus formation in an epicardial coronary artery, resulting in an acute reduction of blood supply to a portion of the myocardium. (See Etiology for details.)
The electrocardiographic (ECG) results of an acute MI are seen below.
Although the clinical presentation of a patient is a key component in the overall evaluation of the patient with MI, many events are either "silent" or are not clinically recognized by patients, families, and health care providers. (See Presentation.) The appearance of cardiac biomarkers in the circulation generally indicates myocardial necrosis and is a useful adjunct to diagnosis. (See Workup.)
MI is considered part of a spectrum referred to as acute coronary syndrome (ACS). The ACS continuum representing ongoing myocardial ischemia or injury consists of unstable angina, non–ST-segment elevation MI (NSTEMI)—collectively referred to as non–ST-segment acute coronary syndrome (NSTE ACS)—and ST-segment elevation MI (STEMI). Patients with ischemic discomfort may or may not have ST-segment or T-wave changes denoted on the electrocardiogram (ECG). ST elevations seen on the ECG reflect active and ongoing transmural myocardial injury. Without immediate reperfusion therapy, most patients with STEMI develop Q waves, reflecting a dead zone of myocardium that has undergone irreversible damage and death.
Those without ST elevations are diagnosed either with unstable angina or NSTEMI―differentiated by the presence of cardiac enzymes. Both these conditions may or may not have changes on the surface ECG, including ST-segment depressions or T-wave morphological changes.
MI may lead to impairment of systolic or diastolic function and to increased predisposition to arrhythmias and other long-term complications.
Coronary thrombolysis and mechanical revascularization have revolutionized the primary treatment of acute MI, largely because they allow salvage of the myocardium when implemented early after the onset of ischemia. (See Treatment.)
The modest prognostic benefit of an opened infarct-related artery may be realized even when recanalization is induced only 6 hours or more after the onset of symptoms; that is, when the salvage of substantial amounts of jeopardized ischemic myocardium is no longer likely. The opening of an infarct-related artery may improve ventricular function and collateral blood flow; prevent ventricular remodeling, as well as decrease infarct expansion, ventricular aneurysm formation, and left ventricular dilatation; and reduce late arrhythmia associated with ventricular aneurysms, and mortality. [5, 6, 7]
Evidence suggests a benefit from the use of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, and statins.
The American College of Cardiology (ACC)/American Heart Association (AHA)/European Society of Cardiology/World Heart Federation released the Observations From the TRITON-TIMI 38 Trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38), which better outlines a universal definition of MI, along with a classification system and risk factors for cardiovascular death. [8]
(See Treatment for more details.)
Definitions
The third universal definition of myocardial infarction
Myocardial infarction (MI), commonly known as a heart attack, is defined pathologically as the irreversible death of myocardial cells caused by ischemia. Clinically, MI is a syndrome that can be recognized by a set of symptoms, chest pain being the hallmark of these symptoms in most cases, supported by biochemical laboratory changes, electrocardiographic (ECG) changes, or findings on imaging modalities able to detect myocardial injury and necrosis.
According to the third universal definition of MI, implemented by a joint task force from the European Society of Cardiology (ESC), American College of Cardiology (ACC) Foundation, American Heart Association (AHA), and the World Heart Federation (WHF), MI is diagnosed when either of the following two criteria are met. [9]
1. Detection of an increase or decrease in cardiac biomarker values (preferably using cardiac troponin [cTn]) with at least one value above the 99th percentile of the upper reference limit (URL) and with at least one of the following findings:
-
Symptoms of ischemia
-
New or presumed new significant ST-segment-T wave (ST-T) changes or new left bundle branch block (LBBB)
-
Development of pathologic Q waves on the ECG
-
Imaging evidence of new loss of viable myocardium or a new regional wall motion abnormality
-
Identification of an intracoronary thrombus by angiography or autopsy
2. Cardiac death with symptoms suggestive of myocardial ischemia and presumed new ischemic changes or injury or new BBB on ECG, but death occurred before cardiac biomarker levles were obtained, or before cardiac biomarker values would be increased.
Types of MI
The Joint ESC/ACCF/AHA/WHF Task Force further classified MI into 5 types on the basis of the underlying cause [7] :
-
Type 1 (spontaneous MI): Related to atherosclerotic plaque rupture, ulceration, fissuring, erosion, or dissection with intraluminal thrombus in one or more of the coronary arteries, leading to decreased myocardial blood flow or distal platelet emboli and thereby resulting in myocyte necrosis. The patient may or may not have underlying obstructive coronary artery disease (CAD).
-
Type 2 (MI secondary to an ischemic imbalance): MI consequent to increased oxygen demand or a decreased supply (eg, coronary endothelial dysfunction, coronary artery spasm, coronary artery embolus, tachyarryhthmias/bradyarrhythmias, anemia, respiratory failure, hypertension, or hypotension).
-
Type 3 (MI resulting in death when biomarker values are unavailable): Sudden, unexpected cardiac death before blood samples for biomarkers could be drawn or before their appearance in the circulation.
-
Type 4a (MI related to percutaneous coronary intervention [PCI]): Elevation of biomarker values (cTn is preferred) to more than 5 times the 99 th percentile of the URL in patients with normal baseline values (< 99 th percentile URL) or a rise of values over 20% if the baseline values are elevated but stable or falling. In addition, any of the following are required: (1) symptoms suggestive of myocardial ischemia; (2) new ischemic ECG changes or new BBB; (3) angiographic loss of patency of a major coronary artery or a side branch or persistent slow flow or no flow or embolization; or (4) demonstration of the new loss of viable myocardium or new regional wall motion abnormality by cardiac imaging.
-
Type 4b (MI related to stent thrombosis): MI associated with stent thrombosis as detected by coronary angiography or autopsy in the setting of myocardial ischemia in combination with a rise and/or fall of cardiac biomarkers with at least one value above the 99 th percentile URL.
-
Type 5 (MI related to coronary artery bypass grafting [CABG]): Elevation of cardiac biomarker values more than 10 times the 99 th percentile URL in patients with normal baseline cTn values. In addition, either (1) new pathologic Q waves or new BBB, (2) angiographic-documented new graft or native coronary artery occlusion, or (3) evidence of new loss of viable myocardium or new regional wall motion abnormality by cardiac imaging is required.
Acute coronary syndrome
The term "acute coronary syndrome" (ACS) refers to a spectrum of conditions that occur due to acute myocardial ischemia and/or infarction as a result of an abrupt reduction in blood flow through the coronary artery circulation.
ACS is divided into two main categories, non–ST elevation (NSTE) ACS and ST-elevation MI (STEMI)
NSTE ACS
NSTE ACS is further divided into unstable angina (UA) and non–ST-elevation myocardial infraction (NSTEMI). These two conditions resemble each other very closely. UA is distinguished from NSTEMI by the absence of an elevation of cardiac biomarker levels. [1]
STEMI
The major discriminating feature of STEMI is the presence of symptoms of myocardial ischemia/injury along with persistent ECG ST-segment elevation in addition to the presence of cardiac biomarkers. [2]
Pathophysiology
Cellular effects of myocardial infarction (MI)
Myocardial injury and myocardial cell death
For the normal heart to continue to function and to steadily pump blood efficiently to meet the demands of the body, it needs to have a constant supply of oxygen and nutrients provided mainly by the coronary circulation. A condition called myocardial ischemia happens if blood supply to the myocardium does not meet the demand. If this imbalance persists, it triggers a cascade of cellular, inflammatory and biochemical events, leading eventually to the irreversible death of heart muscle cells, resulting in MI.
Evolution of MI and ventricular remodeling
The spectrum of myocardial injury depends not only on the intensity of impaired myocardial perfusion but also on the duration and the level of metabolic demand at the time of the event. Severe loss of the ability of the heart muscle cell to contract can be observed as early as within 60 seconds. Persistence of oxygen deprivation to the myocardium through the cessation of blood supply will lead to irreversible myocardial injury within 20 to 40 minutes and up to several hours, depending on several factors including the existing metabolic state of the body and presence of coronary collateral blood flow. [10]
Typical MI initially manifests as coagulation necrosis that is ultimately followed by a healing process characterized by formation of myocardial scarring, known as myocardial fibrosis. This mechanism allows significant architectural changes to the composition, shape and contractile function of the myocardium, especially in the left ventricle, which is the major contributor to the contractile function of the heart. Eventually the left ventricle dilates and changes to a more spherical shape, in a process known as ventricular remodeling. Despite being an irreversible process, ventricular remodeling is a regulated process, therefore, specific treatment strategies and agents should be used in acute MI management in order to reduce the occurrence and severity of ventricular remodeling. [11]
Reperfusion injury
In some occasions, restoration of blood flow to the damaged myocardium triggers further ischemic cellular damage, this paradoxical effect is known as reperfusion injury. This process involves a complex interaction between oxygen free radicals and intracellular calcium, leading to acceleration of myocardial damage and death, microvascular dysfunction and fatal arrhythmias. The role of nitric oxide (an endothelium-derived relaxing factor) as a cardioprotective agent against reperfusion injury, has been demonstrated, as nitric oxide works to inactivate oxygen free radicals, therefore, ameliorating the process of reperfusion injury. [12] Despite the improved understanding of the process of reperfusion injury, there are no specific therapies to prevent it.
Stunned and hibernating myocardium
Stunned myocardium is a condition of transient left ventricular dysfunction following an ischemic event to the myocardium. It occurs if coronary blood flow was impaired for a brief period of time (5 to 15 minutes). Usually, stunned myocardium persists for hours or days following the re-establishment of coronary blood flow.
However, prolonged exposure of the myocardium to an ischemic state, results in an impairment of its contractile function, which can be partial or complete, this is known as myocardial hibernation, and is reversible with revascularization.
Both myocardial stunning and hibernation occur because of loss of essential metabolites required for normal myocardial contractility, such as adenosine, which is needed for adenosine triphosphate (ATP)-dependent contraction. [13]
Plaque
The atheromatous plaque responsible for acute MI develops in a dynamic process in multiple stages. Starting with arterial intimal thickening, which consists of vascular smooth muscles with very minimal or no inflammatory cells, this process can be observed soon after birth. Subsequently, the formation of fibrous cap atheroma occurs, which has a lipid-rich necrotic core that is surrounded by fibrous tissue. Eventually, a thin-cap fibroatheroma develops, this is also known as a vulnerable plaque which is composed mainly of a large necrotic core separated from the vascular lumen by a thin fibrous cap that is infiltrated by inflammatory cells and is deficient of smooth muscle cells, making it vulnerable to rupture. [14, 15]
The process of acute coronary thrombosis leading to ACS involves the pathogenic mechanism of plaque rupture, and less frequently plaque erosion.
The Brasilia Heart Study Group indicates that changes in high-density lipoprotein (HDL) during an MI may alter the antiatherogenic function of HDL to transport lipids from arterial walls. [16] The investigators noted a simultaneous decrease in lipid transfer to HDL and in the capacity of HDL to efflux cholesterol from cells occurs in the acute period after an MI.
In a nested case-control study that evaluated the associations of plasma metabolic markers with the risks of incident MI, ischemic stroke, and intracerebral hemorrhage, investigators found positive associations of lipoproteins and lipids with MI and ischemic stroke but not with intracerebral hemorrohage, as well as positive associations between triglyceride concentrations and MI. [17] Except for small HDL, there was also an inverse association of HDL particles with MI, and an inverse association of cholesterol in large HDL with MI and ischemic stroke. The study cohort included 912 patients with MI, 1146 with ischemic stroke, 1138 with intracerebral hemorrhage, and 1466 control subjects. [17]
Etiology
Atherosclerosis is the disease primarily responsible for most acute coronary syndrome (ACS) cases. Approximately 90% of myocardial infarctions (MIs) result from an acute thrombus that obstructs an atherosclerotic coronary artery. Plaque rupture and erosion are considered to be the major triggers for coronary thrombosis. Following plaque erosion or rupture, platelet activation and aggregation, coagulation pathway activation, and endothelial vasoconstriction occur, leading to coronary thrombosis and occlusion.
Within the coronary vasculature, flow dynamics and endothelial shear stress are implicated in the pathogenesis of vulnerable plaque formation. [18] A large body of evidence indicates that in numerous cases, culprit lesions are stenoses of less than 70% and are located proximally within the coronary tree. [19, 20] Coronary atherosclerosis is especially prominent near branching points of vessels. [21] Culprit lesions that are particularly prone to rupture are atheromas containing abundant macrophages, a large lipid-rich core surrounded by a thinned fibrous cap.
Nonmodifiable risk factors for atherosclerosis include the following:
-
Age
-
Sex
-
Family history of premature coronary heart disease
-
Male-pattern baldness
Modifiable risk factors for atherosclerosis include the following [22] :
-
Smoking or other tobacco use
-
Hypercholesterolemia and hypertriglyceridemia, including inherited lipoprotein disorders
-
Dyslipidemia
-
Diabetes mellitus
-
Hypertension
-
Obesity (abdominal obesity)
-
Psychosocial stress
-
Sedentary lifestyle and/or lack of exercise
-
Reduced consumption of fruits and vegetables
-
Poor oral hygiene
-
Type A personality
-
Elevated homocysteine levels
-
Presence of peripheral vascular disease
MI can also occur for causes other than atherosclerosis. Nonatherosclerotic causes of MI include the following:
-
Coronary occlusion secondary to vasculitis
-
Ventricular hypertrophy (eg, left ventricular hypertrophy, hypertrophic cardiomyopathy)
-
Coronary artery emboli, secondary to cholesterol, air, or the products of sepsis
-
Coronary trauma
-
Primary coronary vasospasm (variant angina)
-
Drug use (eg, cocaine, amphetamines, ephedrine)
-
Arteritis
-
Coronary anomalies, including aneurysms of coronary arteries
-
Factors that increase oxygen requirement, such as heavy exertion, fever, or hyperthyroidism
-
Factors that decrease oxygen delivery, such as hypoxemia of severe anemia
-
Aortic dissection, with retrograde involvement of the coronary arteries
In addition, MI can result from hypoxia due to carbon monoxide poisoning or acute pulmonary disorders.
Although rare, pediatric coronary artery disease may be seen with Marfan syndrome, Kawasaki disease, Takayasu arteritis, progeria, and cystic medial necrosis.
Acute MI is rare in childhood and adolescence. Although adults acquire coronary artery disease from lifelong deposition of atheroma and plaque, which causes coronary artery spasm and thrombosis, children with acute MI usually have either an acute inflammatory condition of the coronary arteries or an anomalous origin of the left coronary artery. Intrauterine MI also does occur, often in association with coronary artery stenosis. [25]
Epidemiology
United States statistics
Coronary artery disease (CAD) is the leading cause of death in the United States; approximately 500,000-700,000 deaths related to CAD occur each year, making it the cause of death in an estimated one third of all deaths in the population for those older than 35 years.
Approximately 1.5 million cases of myocardial infarction (MI) occur annually in the United States; the yearly incidence rate is approximately 600 cases per 100,000 people. The proportion of patients diagnosed with non–ST-elevation MI (NSTEMI) compared with ST-elevation MI (STEMI) has progressively increased. Despite an impressive decline in age-adjusted death rates attributable to acute MI since the mid-1970s, the total number of MI-related deaths in the United States has not declined. [26]
The death rate related to acute MI is approximately three times higher in men than in women. It is more frequent in black patients compared to white patientss, an excess that disappears by age 75 years. Among the Hispanic population, coronary mortality is not as high as it is among black individuals and white persons. [27]
European statistics
CAD is also the number one cause of death in European countries.
In the European Union, death rates related to CAD dropped by almost 30% between the mid 1960s to the mid and late 1990s; however, within Eastern European countries, there was an increase in death rates related to acute MI in the early1990s, followed by a subsequent decline. In the Russian Federation, cardiovascular mortality remained the same. [28]
Cardiovascular disease in other developed countries and in developing nations
An analysis of death certificates from the World Health Organization (WHO) database demonstrated that CAD mortality in Japan was significantly lower than in the United States and Europe, and it was further reduced by about 30% by the mid 1990s. [28]
In China, there has been a significant increase in mortality related to CAD, this is most likely attributed to the increase in cardiovascular disease risk factors, predominantly smoking and dyslipidemia. [29]
The incidence of CAD and related mortality is expected to rise dramatically in other developing countries including India, Latin America, the Middle East and Sub-Saharan Africa, with an estimated 80% increase, from approximately 9 million in 1990 to a projected 20 million by 2020. [30, 31]
It is believed that these international trends in the incidence of CAD and subsequent acute MI are largely related to consequences of social and economic changes in these countries, resulting in better healthcare access and increases in life expectancy, in addition to adoption of westernized diets, reduction in physical activity, and higher rates of smoking.
A major Canadian-led global study (INTERHEART trial) in 52 countries across Africa, Asia, Australia, Europe, the Middle East, and North and South America, has identified 9 easily measured risk factors (smoking, abnormal blood lipid levels, hypertension, diabetes, obesity, diet, physical activity, alcohol consumption, and psychosocial factors) that account for over 90% of the risk for acute MI. [22] The INTERHEART investigators found that these risk factors are the same in almost every geographic region and every racial/ethnic group worldwide, and they are consistent in men and women. The INTERHEART trial showed that smoking 1-5 cigarettes daily increased the risk of an acute MI by 40%, and the risk increased with the amount of tobacco smoked per day. It also concluded that all forms of tobacco, including filtered and nonfiltered cigarettes, pipes and cigars, and chewing tobacco, are harmful, and that abdominal obesity is a greater risk factor than body-mass index (BMI), indicating that measurement of waist-to-hip ratio could replace BMI as an indicator of obesity. [22, 31, 32]
Prognosis
Acute myocardial infarction (MI) is associated with a 30% mortality rate; about 50% of the deaths occur prior to arrival at the hospital. An additional 5-10% of survivors die within the first year after their myocardial infarction. Approximately half of all patients with an MI are rehospitalized within 1 year of their index event.
Overall, prognosis is highly variable and depends largely on the extent of the infarct, the residual left ventricular function, and whether the patient underwent revascularization.
Better prognosis is associated with the following factors:
-
Successful early reperfusion (ST-elevation MI [STEMI] goals: patient arrival to fibrinolysis infusion within 30 minutes OR patient arrival to percutaneous coronary intervention [PCI] within 90 minutes)
-
Preserved left ventricular function
-
Short-term and long-term treatment with beta-blockers, aspirin, and angiotensin-converting enzyme (ACE) inhibitors
Poorer prognosis is associated with the following factors:
-
Advanced age
-
Diabetes mellitus
-
Previous vascular disease (eg, cerebrovascular disease or peripheral vascular disease)
-
Elevated thrombolysis in MI (TIMI) risk score for unstable angina/non–ST elevation acute coronary syndrome (NSTE-ACS) (TIMI risk score includes 7 factors: age ≥65 y, ≥3 risk factors for cardiac disease, previous coronary disease, ST-segment deviation ≥0.5 mm, ≥2 episodes of angina in last 24 hours, aspirin use within prior week, and elevated cardiac enzyme levels) [1, 3, 33]
-
Delayed or unsuccessful reperfusion
-
Poorly preserved left ventricular function (the strongest predictor of outcome)
-
Elevated high sensitive C-reactive protein (hs-CRP), a nonspecific inflammatory marker [39]
-
Depression
It has been shown that five baseline parameters at presentation of patients with acute MI account for over 90% of the prognostic predictors of 30-day mortality from acute MI. These parameters include age, systolic blood pressure on presentation, Killip classification, heart rate, and anatomic location of the MI.
Killip classification
The Killip classification is widely used in patients presenting with acute MI for the purpose of risk stratification, as follows [42] :
-
Killip class I includes individuals with no clinical signs of heart failure
-
Killip class II includes individuals with rales or crackles in the lungs, an S 3 gallop, and elevated jugular venous pressure
-
Killip class III describes individuals with frank acute pulmonary edema
-
Killip class IV describes individuals in cardiogenic shock or hypotension (measured as systolic blood pressure < 90 mmHg), and evidence of low cardiac output (oliguria, cyanosis, or impaired mental status).
Patient Education
Patients with active symptoms of acute coronary syndrome (ACS) should be instructed to call emergency services (eg, 911 in the United States), and they should be transported by emergency medical services personnel, not by themselves, family, or friends. Patients should be instructed to go to the emergency department immediately if the suspected ACS symptoms last longer than 20 minutes at rest or are associated with near syncope/syncope or hemodynamic instability.
If nitroglycerin is prescribed to a patient with suspected ACS, the patient should be instructed to take a dose if symptoms arise. If no relief is experienced 5 minutes after the first dose, the patient should contact emergency services. If relief is experienced within 5 minutes of the first nitroglycerin dose, repeated doses can be given every 5 minutes for a maximum of 3 doses total. If by then the symptoms have not yet fully resolved, the patient, a family member, or a caregiver should contact emergency services. [1]
Diet plays an important role in the development of coronary artery disease (CAD). Educate post–myocardial infarction (MI) patients about the role of a low-cholesterol and low-salt diet. A dietitian should see and evaluate all patients prior to discharge from the hospital. Additionally, emphasis on exercise training should be made, because current evidence demonstrates that cardiac rehabilitation after MI results in lower rates of recurrent cardiovascular events. [43]
All patients should be educated regarding the critical role of smoking in the development of CAD. Smoking cessation classes should be offered to help patients avoid smoking after their MI.
For patient education resources, see the Heart Health Center and Cholesterol Center, as well as High Cholesterol, Cholesterol Charts (What the Numbers Mean), Lifestyle Cholesterol Management, Chest Pain, Coronary Heart Disease, Heart Attack, Angina Pectoris, Cholesterol-Lowering Medications, and Statins for Cholesterol.
-
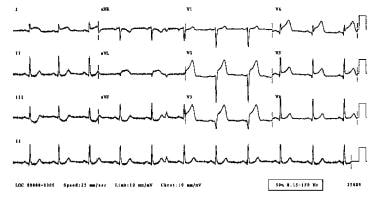
Acute anterior myocardial infarction.
-
Acute inferior myocardial infarction.
-
Acute posterolateral myocardial infarction.
-
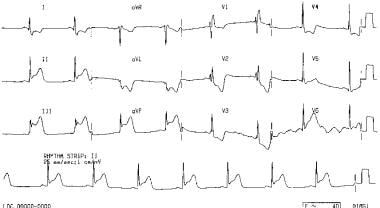
A 53-year-old patient who had experienced 3 hours of chest pain had a 12-lead electrocardiogram performed, and the results are as shown. He was given sublingual nitroglycerin and developed severe symptomatic hypotension. His blood pressure normalized with volume resuscitation.
-
The right-sided leads indicate ST-segment elevations in RV<inf>3</inf> to RV<inf>5</inf>, which are consistent with a right ventricular infarct.
-
Timing of release of various cardiac biomarker peaks after the onset of myocardial infarction
-
Modified 2-dimensional (top) echocardiogram and color flow Doppler image (bottom). Apical 4-chamber views show a breach in the interventricular septum and free communication between ventricles through a large apical septum ventricular septal defect in a patient who recently had an anterior myocardial infarction.
-
Apical 2-chamber view depicts a large left ventricular apical thrombus with mobile extensions.
-
Parasternal long-axis view of the left ventricle demonstrates a large inferobasal aneurysm. Note the wide neck and base of the aneurysm.
-
Acute myocardial infarct. At 3 days, there is a zone of yellow necrosis surrounded by darker hyperemic borders. The arrow points to a transmural infarct in the posterior wall of the left ventricle, in this short axis slice through the left and right ventricular chambers.
-
Acute myocardial infarction, reperfusion type. In this case, the infarct is diffusely hemorrhagic. There is a rupture track through the center of this posterior left ventricular transmural infarct. The mechanism of death was hemopericardium.
-
Healing myocardial infarction, lateral left ventricle. In this heart, there is a variegated or mottled appearance to the lateral left ventricle (left). This infarct began 19 days prior to death.
-
Early healed myocardial infarction, anterior septum. There is a glistening gelatinous appearance to this infarction, which occurred 6 weeks prior to death, from embolization during valve surgery.
-
Healed myocardial infarction, anterior left ventricle. There is diffuse scarring (white) with marked thinning of the ventricle (aneurysm).
-
Acute myocardial infarct. The earliest change is hypereosinophilia (above) with an intense pink cytoplasm. There is no inflammation at border between the necrotic myocardium and the viable myocardium (left and below), indicating that the necrosis is about 12-24 hours in age.
-
Acute myocardial infarct. After 24 hours, there is a neutrophilic infiltrate at the border of the infarct. Viable myocardium is at the left, and neutrophils with apoptosis (karyorrhexis) are seen infiltrating the necrotic muscle. This patient experienced abdominal pain 35 hours prior to death.
-
Healing myocardial infarct. This patient died 8 days after experiencing sudden chest pain at rest. There is a large area of necrosis with hypereosinophilia of myocytes, with a rim of viable myocardium at the very bottom. At the border, there is chronic inflammation with early granulation tissue, with ingrowth of endothelial cells.
-
Healing myocardial infarct. At 10 days to 2 weeks, there is chronic inflammation, hemosiderin-laden macrophages, and early fibroblasts without significant collagen deposition.
-
Healed myocardial infarct. At 3 months, there is dense scar, which is blue on this Masson trichrome stain. This infarct was subendocardial, in the posterior left ventricle near the ventricular septum.
-
This is a posteroanterior view of a right ventricular endocardial activation map during ventricular tachycardia in a patient with a previous septal myocardial infarction. Earliest activation is recorded in red; late activation shows as blue to magenta. Fragmented low-amplitude diastolic local electrocardiograms were recorded adjacent to the earliest (red) breakout area, and local ablation in this scarred zone (red dots) resulted in termination and noninducibility of this previously incessant arrhythmia.
-
A color-enhanced angiogram of the heart left shows a plaque-induced obstruction (top center) in a major artery, which can lead to myocardial infarction (MI). MIs can precipitate heart failure.