Background
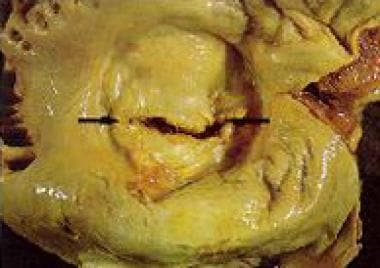
Mitral stenosis (MS) is a narrowing of the inlet valve into the left ventricle that prevents proper filling during diastole. Patients with mitral stenosis typically have mitral valve leaflets that are thickened, commissures that are fused, and/or sub-valvular structures that are thickened and shortened. See the image below.
The most common cause of mitral stenosis is rheumatic fever (RF). Indeed, rheumatic involvement is found in 99% of stenotic mitral valves evaluated at the time of valve replacement. Although rheumatic fever is exceedingly rare today in the United States, the prevalence and consequent morbidity remains significant in impoverished populations and developing countries.
In the United States and other developed countries, the progression to mitral stenosis is typically slow, with the onset of symptoms following a latency period of 20-40 years. Once symptoms present, an acceleration of disease may occur. Conversely, in developing countries, rheumatic heart disease can be frequently diagnosed at school age and symptoms often present during the teenage years. [1] The progression of mitral stenosis occurs rapidly relative to the natural history in developed countries; moderate-to-severe disease requiring surgical or endovascular intervention is not uncommon in the teenage years or young adulthood. This early presentation and accelerated course is theorized to be a consequence of recurrent, untreated rheumatic fever.
Pathophysiology
Nearly all mitral stenosis is secondary to the sequelae of rheumatic fever, especially in the developing world. Other causes are rare and include mucopolysaccharoidoses, severe annular calcification, congenital deformities (which usually require intervention in infancy or early childhood), diseases of serotonin metabolism, methysergide therapy, and systemic autoimmune disease (eg, systemic lupus erythematous, rheumatoid arthritis). [2, 3] Left atrial myxoma, ball-valve thrombus, infective endocarditis, and cor triatriatum may mimic mitral stenosis.
Development of rheumatic fever, the autoimmune disease that is the most common cause of mitral stenosis, requires both a genetically susceptible individual and infection by specific rheumatogenic strains of Group A Streptococcus (GAS). [4, 5, 6, 7, 8] Antibodies produced against the M proteins of these certain strains of GAS cross-react with cardiac tissue. [7] Pathologic examination of the mitral valve at this time reveals proliferation of fibroblasts and macrophages.
Subsequent valvular stenosis may occur as a consequence of the healing of the rheumatic process, repetitive but subclinical rheumatic insults or reinfection, chronic rheumatic activity, or progressive hemodynamic stresses on the traumatized valve, similar to that of the pathogenesis of aortic stenosis. The plethora of postulated mechanisms and contexts for this pathologic evolution may account for the fact that some patients experience a chronic stable disease, whereas others have an accelerated course necessitating early surgical intervention. [9, 10]
The normal area of the mitral valve orifice is 4-6 cm2. [2, 3] This effectively creates a common chamber between the left atrium and the left ventricle in diastole. In early diastole, a small and brief pressure gradient is present; however, during most of the filling period, the pressures in the two chambers are equal. Narrowing of the valve area to less than 2.5 cm2 impedes the free flow of blood and requires increased left atrial pressure (LAP) to ensure normal transmitral flow.
Symptoms typically first present after exertion when the valve area shrinks to less than 2.5 cm2, and symptoms at rest do not begin until valve area reaches 1.5 cm2. [2, 3] However, any physiologic stress requiring increased cardiac output (eg, pregnancy, infection, exercise, emotional stress, anemia, atrial fibrillation with rapid ventricular response) may precipitate symptoms earlier in the progression of stenosis.
Severe mitral stenosis occurs when the opening is reduced to 1 cm2. At this stage, a mean LAP of 25 mm Hg is required to maintain a normal cardiac output. With progressive stenosis, critical flow restriction reduces left ventricular preload and output. The increase in LAP also enlarges the left atrium and raises pulmonary vascular pressures. The resulting pulmonary congestion and reduced cardiac output can mimic primary left ventricular failure. However, left ventricular contractility is normal in most cases of isolated mitral stenosis. [11, 10] As the disease evolves, chronic elevation of the LAP eventually leads to pulmonary hypertension, tricuspid and pulmonary valve incompetence, and secondary right heart failure.
Etiology
Causes of mitral stenosis include the following:
-
Rheumatic fever (most common, all others are rare)
-
Congenital mitral stenosis
-
Systemic lupus erythematosus (SLE)
-
Rheumatoid arthritis (RA)
-
Malignant carcinoid
-
Mucopolysaccharidoses (of the Hunter-Hurler phenotype)
-
Fabry disease
-
Whipple disease
Epidemiology
United States data
The prevalence of mitral stenosis in the United States has concurrently decreased with the dramatic decline in rheumatic fever. The reason for the decreased incidence of rheumatic fever is likely multifactorial. Antibiotic therapy may be partially responsible, but some authors have noted that the decline began before antibiotics were “widely available.” [10] The population with Group A Streptococcus (GAS) has also changed. The fraction of strains that have been identified as “rheumatogenic” has decreased in developed countries with a concomitant decreasing incidence of rheumatic fever. [12, 8] Additionally, evidence suggests that improved socioeconomic status, public health, and hygiene have resulted in the near disappearance of rheumatic fever and new cases of mitral stenosis in the United States and other developed countries. [13]
The true prevalence of mitral stenosis is unknown and rheumatic fever is no longer a disease that must be reported to the Centers of Disease Control and Prevention (CDC). However, approximately 1500 balloon mitral valvotomies were performed in the United States during 2004. [10] This provides a rough indication of the prevalence of moderate to severe disease. Nkomo et al used echocardiography to prospectively study all valvular disease in the United States and estimated the prevalence of mitral stenosis at 0.1% (0.02-0.2%). [14]
International data
Both rheumatic fever and mitral stenosis remain common in developing countries. Mitral stenosis develops at an earlier age, progresses more quickly, and requires earlier intervention. [3, 15]
Attempts to estimate the prevalence of rheumatic heart disease and mitral stenosis in the developing world have been hindered by inconsistent diagnostic criteria and methods of diagnosis, limited access to appropriate diagnostic tools, and under-reporting. [16, 17, 18, 19, 20] The reported prevalence of rheumatic heart disease in a recent study of randomly selected schoolchildren in southeast Asia and sub-Saharan Africa using both Echo-Doppler and echocardiographic morphologic criteria was reported to be 30.4 cases per 1000 children in Mozambique and 21.5 cases per 1000 children in Cambodia. [18] However, only 15-20% of the total rheumatic heart disease in a given population is in schoolchildren. [13, 19] Therefore, even the estimates in Mozambique and Cambodia, using up-to-date modalities and diagnostic criteria, may underestimate true prevalence.
Sex- and age-related demographics
The incidence of rheumatic fever is nearly equal in males and females, but mitral stenosis develops 2-3 times more frequently in females. [10]
In developed countries, the initial symptomatic presentation of mitral stenosis is usually in the fourth to sixth decades of life. [21] Presentation is thought to occur after a latency period of 20-40 years after rheumatic fever. In contrast, patients in the developing world have a more quickly progressive coarse and often present with symptomatic mitral stenosis in the late teenage years or in early adulthood. [3]
Prognosis
Overall, the 10-year survival rate in untreated patients is 50-60%. [22, 23] Cause of death in untreated patients is due to congestive cardiopulmonary failure (60-70%), systemic embolism (20-30%), pulmonary embolism (about 10%), and infection (1-5%). Of note, patients with mitral stenosis have inherent hypercoagulability independent of atrial rhythm. [24]
Predicted mortality depends on symptom severity at presentation. Asymptomatic or mildly symptomatic patients have a survival rate of 80% or higher at 10 years, whereas in the severely symptomatic patient, survival is 0-15% at 10 years.
Depending on severity of symptoms of mitral stenosis, the 10-year survival rate is as follows:
-
85% for no symptom (class I)
-
34-42% for mild symptoms (early class II)
-
40% for moderate-severe symptoms (late class II, class III)
-
0% for class IV (Of class IV patients, survival is 42% at 1 year and 10% at 5 years.)
The operative mortality rate is 1-2% for mitral commissurotomy and 2-5% for mitral valve replacement.
Complications
Complications of mitral stenosis may include all of the following:
-
Thromboembolism
-
Atrial fibrillation
-
Bacterial endocarditis
-
Pulmonary hypertension
-
Pulmonary edema
-
Complications of balloon valvulotomy (eg, stroke, cardiac perforation, development of mitral regurgitation)
-
Complications of mitral valve replacement (eg, perivalvular leak, thromboembolism, infective endocarditis, mechanical dysfunction, bleeding due to anticoagulants)
-
Mitral stenosis.
-
Echocardiography of mitral stenosis. Courtesy of Michael B. Stone, MD, RDMS.