Practice Essentials
Metabolic acidosis is a clinical disturbance characterized by an increase in plasma acidity. Metabolic acidosis should be considered a sign of an underlying disease process. Identification of this underlying condition is essential to initiate appropriate therapy. [1] The initial therapeutic goal for patients with severe acidemia is to raise the systemic pH above 7.1-7.2, a level at which dysrhythmias become less likely and cardiac contractility and responsiveness to catecholamines will be restored. [2, 3]
This article discusses the differential diagnosis of metabolic acidosis and presents a scheme for identifying the underlying cause of acidosis by using laboratory tests that are available in the emergency department. Clinical strategies for treating metabolic acidosis are also reviewed.
Signs and symptoms of metabolic acidosis
Metabolic acidosis can result in a variety of nonspecific changes in several organ systems, including, but not limited to, neurologic, cardiovascular, pulmonary, gastrointestinal, and musculoskeletal dysfunction. Symptoms are often a result of and specific to the underlying etiology of the metabolic acidosis.
Neurologic signs and symptoms include the following:
-
Cranial nerve palsies may occur with ethylene glycol intoxication
-
Retinal edema may be seen in methanol ingestions
-
Lethargy, stupor, and coma may occur in severe metabolic acidosis, particularly when it is associated with a toxic ingestion
With regard to the cardiovascular system, severe acidemia (ie, pH < 7.10) can predispose a patient to potentially fatal ventricular arrhythmias, and it can reduce cardiac contractility and the inotropic response to catecholamines, resulting in hypotension and congestive heart failure.
Pulmonary signs and symptoms include the following:
-
Patients with acute metabolic acidosis demonstrate tachypnea and hyperpnea as prominent physical signs
-
Kussmaul respiration, an extremely intense respiratory effort, may be present
-
Hyperventilation, in the absence of obvious lung disease, should alert the clinician to the possibility of an underlying metabolic acidosis
With regard to the musculoskeletal system, chronic metabolic acidosis (eg, uremia, renal tubular acidosis [RTA]) is associated with substantial bone disease from bone buffering of calcium carbonate. [4]
Workup in metabolic acidosis
Lab studies in the workup of metabolic acidosis include the following:
-
Arterial blood gas analysis - A low HCO 3 level found on an automated sequential multiple analyzer (SMA) (eg, serum chemistries) is often the first clue to the presence of metabolic acidosis
-
Serum chemistry
-
Complete blood count (CBC)
-
Urinalysis - If the urine pH is above 5.5 in the face of acidemia, this finding is consistent with a type I RTA
Other tests include the following:
-
Anion gap (AG)
-
Ketone levels
-
Serum lactate levels
-
Salicylate levels
-
Iron levels
Management of metabolic acidosis
The initial therapeutic goal for patients with severe acidemia is to raise the systemic pH above 7.1-7.2, a level at which dysrhythmias become less likely and cardiac contractility and responsiveness to catecholamines will be restored.
Treating the underlying conditions in high AG states usually is sufficient to reverse the acidosis. Treatment with bicarbonate is unnecessary, except in extreme cases of acidosis when the pH is less than 7.1-7.2. For all cases of diabetic ketoacidosis, the role of bicarbonate is controversial, regardless of the pH or bicarbonate level.
In hyperchloremic acidosis, the central problem is with the reabsorption or regeneration of bicarbonate. In these conditions, therapy with bicarbonate makes physiologic sense and is prudent in patients with severe acidosis.
Pathophysiology
There are 3 approaches to understanding acid/base balance: A qualitative approach using the Henderson/Hasselbalch equation, a semiqualitative approach with base excess, and the Strong Ion Theory. The 3 theories are reviewed below.
Henderson-Hasselbalch approach to acid/base physiology
The Henderson-Hasselbalch equation describes the relationship between blood pH and the components of the H2CO3 buffering system. This qualitative description of acid/base physiology allows the metabolic component to be separated from the respiratory components of acid/base balance.
pH = 6.1 + log (HCO3/H2CO3)
Bicarbonate (HCO3) is in equilibrium with the metabolic components.
-
Bicarbonate production in the kidney
-
Acid production from endogenous or exogenous sources
Carbonic acid (H2CO3) is in equilibrium with the respiratory component, as shown by the below equation:
H2CO3 = PCO2 (mm Hg) X 0.03
Metabolic acidosis can be caused by the following:
-
Increase in the generation of H+ from endogenous (eg, lactate, ketones) or exogenous acids (eg, salicylate, ethylene glycol, methanol)
-
Inability of the kidneys to excrete the hydrogen from dietary protein intake (type I, IV renal tubular acidosis)
-
The loss of bicarbonate (HCO3) due to wasting through the kidney (type II renal tubular acidosis) or the gastrointestinal tract (diarrhea)
-
The kidneys' response to a respiratory alkalosis
Base excess approach to acid/base physiology
Unfortunately, the Henderson/Hasselbalch equation is not linear; pCO2 adjusts pH as part of the normal respiratory compensation for acid/base derangements. This nonlinearity of Henderson-Hasselbalch prevents this equation from quantifying the exact amount of bicarbonate deficit in a metabolic acidosis. This observation led to the development of a semiquantitative approach, base excess (BE).
BE = (HCO3 – 24.4 + [2.3 X Hgb + 7.7] X [pH – 7.4]) X (1 – 0.023 X Hgb)
Base excess attempts to give a quantitative amount of bicarbonate (mmol) that is required to be added or subtracted to restore 1 L of whole blood to a pH of 7.4 at a pCO2 of 40 mm Hg. To standardize BE for hemoglobin, the following formula was developed with improved in vivo accuracy, the standardized base excess (SBE):
SBE = 0.9287 X (HCO3 – 24.4 + 14.83 X [pH – 7.4])
Strong Ion approach to acid/base physiology
These classical descriptions of acid/base physiology often failed to account for acid/base findings in critically ill patients. An alkalosis was often noted in critically ill patients as their serum albumin level decreased, which could not be quantified by Henderson Hasselbalch or BE. Also, the "dilutional" acidosis frequently encountered after a large infusion of normal saline could not be explained by either of these 2 approaches to acid/base balance.
Both Henderson Hasselbalch and BE assume that the cations (Ca2+, Mg2+) and anions (Cl-, albumin, PO4-) in plasma remain unchanged in a patient with metabolic acidosis. Yet, in critically ill patients, these ions are known to be in dynamic flux. During the 1980s, Dr. Peter Stewart developed an acid/base theory (Strong Ion) using quantitative chemistry, which accounted for fluctuations of all the ions dissolved in plasma. Based on the requirements for electrical neutrality in any solution as any one of the concentrations of these ions changes, water must dissociate into H+ or OH- to balance the charge. The pH in this scheme is not a consequence of the ratio of acid to base in solution but determined by 3 independent variables:
-
Strong ion difference (SID) – Ions almost completely dissociated at physiologic pH.
SID = [Na+ + K+ + Ca2+ + Mg2+] – [Cl- + Lactate-]
(Ca2+ and Mg2+ are the concentrations of their ionized forms, Mg2+ X 0.7 = ionized Mg2+ concentration)
-
Total weak acid concentration (Atot) – Ions that can exist dissociated (A-) or associated (AH) at physiologic pH (buffers)
Atot = 0.325275 X [albumin] + 2 X [phosphate]
-
pCO 2 (mm Hg)
The Henderson Hasselbalch equation can be reformulated with variables from the Strong Ion Theory to give a more generalizeable solution to pH.
pH = pK1 ’ + log [SID] – Ka – [ATOT]/[Ka + 10–pH]
SPCO2
(K1’ is the equilibrium constant for the Henderson-Hasselbalch equation, Ka is the weak acid dissociation constant, and S is the solubility of CO2 in plasma.) See the image below.
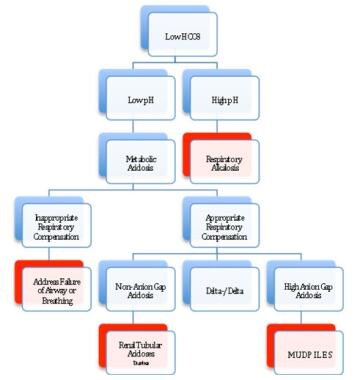
Once a metabolic acidosis is suspected by low bicarbonate concentration, an arterial blood gas analysis should be obtained. The low HCO3 level can be caused either by a primary metabolic acidosis or as the metabolic compensation for a respiratory alkalosis. The direction of the pH will separate metabolic acidosis (pH < 7.35) from a respiratory alkalosis (pH > 7.45).
The normal respiratory response (Kussmaul breathing) to a metabolic acidosis is a decrease in pCO2. This is given by the Winter’s equation:
PCO2 = 1.5 X (observed HCO3) + 8±2
(A quick rule of thumb: The PCO2 should approximate the last two digits of pH. For example, pH 7.25, PCO2 should be close to 25 mm Hg.)
Failure to have an appropriate respiratory response to metabolic acidosis represents a failure of airway and/or breathing, which must be addressed before any other workup commences.
Once an appropriate respiratory response for a metabolic acidosis has been established, the workup for the presence of unmeasured anions can progress by using the traditional anion gap, the delta-delta approach, or the strong ion gap. This allows the differential of metabolic acidosis to be narrowed and the appropriate therapy applied.
To differentiate between the causes of metabolic acidosis, one traditionally calculates the anion gap (AG), corresponding the presence of unmeasured anions. [5]
AG = (Na+) - ([Cl-] + [HCO3-])
The anion gap allows for the differentiation of 2 groups of metabolic acidosis. Metabolic acidosis with a high AG is associated with the addition of endogenously or exogenously generated acids. Metabolic acidosis with a normal AG is associated with the loss of HCO3 from the kidney or GI tract, or the failure of the kidney to excrete H+.
The delta/delta concept allows for the partitioning of metabolic acidosis into an anion gap and a non-anion gap component, which can occur contemporaneously. The concept behind delta/delta is based on the assumption that for every increase in anion gap of 1 mmol/L above normal (12 mmol), serum HCO3- will drop by an equal amount. [6]
Δ anion gap = Δ HCO3
If the delta HCO3- is greater than the delta anion gap, then a concomitant non-anion gap acidosis must exist along side the anion gap acidosis. One example would be a patient with a congenital renal tubular acidosis in diabetic ketoacidosis (DKA).
Stewart provides a replacement for the standard anion gap and delta/delta, which allows one to directly measure the amount of unmeasured anions in solution corrected for changes from normal of Ca2+, Mg2+, albumin, and phosphate. [7] This is the Strong Ion Gap (SIG). All the strong ions are expressed in mEq/L, and only the ionized portions of Mg2+ and Ca2+ are considered (to convert total to ionized Mg2+, multiply by 0.7). Because of the complexity of the equation, several Internet resources are available to calculate the SIG. For example, The Stewart approach to acid-base is a good resource. The normal SIG is between 0 and 2. SIG has been shown to be better than blood lactate, pH, or injury severity scores in trauma patients and pediatric surgery patients as a predictor of mortality.
A report by Masevicius and Dubin stated, however, that the variables presumed to be independent in the Stewart approach are actually interdependent in various situations and that there is a lack of experimental evidence to show that water dissociates in response to SID changes. The report also contends that severe methodologic drawbacks exist in studies that have attempted to demonstrate that the Stewart approach is clinically superior to conventional methods for analyzing acid-base disorders, while the largest such study indicated that the Stewart approach can be used interchangeably with conventional techniques. Masevicius and Dubin concluded that the Stewart approach does not offer a significantly better method of understanding, diagnosing, and treating acid-base changes in critically ill individuals. [8]
Prognosis
Because metabolic acidosis is a condition that occurs in response to a variety of disease states, the prognosis is directly related to the underlying etiology and the ability to treat or correct that particular disorder.
A study by Raikou indicated that in patients undergoing renal replacement therapy, an association exists between uncorrected severe metabolic acidosis (serum bicarbonate concentrations of below 20 mmol/L) and a 10-year risk for coronary heart disease of over 20%, as well as a high overall mortality rate. [9]
A study by Park et al indicated that a high rate of metabolic acidosis occurs in kidney transplant recipients; a low serum total CO2 concentration (< 22 mmol/L) was found in about 30-70% of such patients with an estimated glomerular filtration rate of under 30 mL/min per 1.73 m2. The study also found evidence that metabolic acidosis may increase the likelihood of graft failure and mortality in kidney transplant recipients. [10]
In a study of emergency department patients with acute kidney injury, Safari et al determined, through multivariate analysis, that metabolic acidosis is independently associated with mortality, along with sex, age over 60 years, blood urea nitrogen (BUN) concentration, hyperkalemia, cause of renal failure, and type of renal failure. [11]
-
Approach for evaluating metabolic acidosis.