Overview
Malaria is the most deadly vector-borne human disease in the world. [1] Although typically an illness of tropical regions of the world, more than 2000 cases of malaria are diagnosed in the United States each year, with nearly all originating from outside the country. Of these, approximately 300 patients have severe disease. [2] In Latin America, malaria is known as paludismo. Blackwater fever refers malarial hemoglobinuria, which is characterized by dark urine. This occurs in some cases of malaria and results from severe red blood cell (RBC) hemolysis. [3]
There are over 120 Plasmodium species identified as capable of infecting mammals, birds, and reptiles. Of these there are six species that most regularly infect humans. P falciparum and P knowlesi cause the most severe morbidity and mortality, particularly among African children. Plasmodium vivax typically causes relatively milder disease. Relatively less is known about Plasmodium ovale curtisi and Plasmodium ovale wallikeri, however disease is typically of similar course and severity of vivax malaria. Plasmodium knowlesi is primarily in Southwest Asia.
The infection that causes malaria is transmitted exclusively by mosquitos in the genus Anopheles. In addition to infecting mosquitos and humans, other primates, bats, birds, and lizards can also be infected. With increasingly active worldwide travel, urbanization, travel to remote endemic areas, and human interaction with wild animals, humans are becoming more exposed to the risk for zoonotic diseases. Although the general global trend of malaria has decreased and stabilized over the last few decades, [4] there is an anticipation that global warming will increase the incidence of malaria worldwide. Proposed mechanisms include increased global temperatures causing an accelerated plasmodium life cycle, as well as increased rain in endemic regions providing larger capacity for the Anopheles mosquito habitat. It is estimated that Africa and Southeast Asia will be the most effected regions in this regard, and that there is an estimated 20% increase in mortality of children aged 0–4 in the worst hit regions by the end of the 21st century. [5]
The World Health Organization (WHO) reports that there are 97 countries in which malarial disease transmission occurs, with 40% of the world’s population at risk for infection and hundreds of millions of cases and hundreds of thousands of deaths annually, mostly in children, and over 90% in sub-Saharan Africa.
The emergency physician practicing in what are typically considered nonendemic countries, such as the United States, should have a high index of suspicion for malaria and other infectious zoonotic diseases, including other hemorrhagic fevers (eg, dengue or, less commonly, Ebola virus infection), in patients who present with a history of fever and travel or immigration from an endemic region. Viral hemorrhagic fever may present similarly.
Failure to consider malaria in the differential diagnoses of a febrile illness following such travel, even if seemingly temporally remote, and even when antimalarial prophylaxis medications have been reportedly taken as directed, can result in significant morbidity or mortality, especially in children and pregnant or immunocompromised patients. Clinical relapse with infections due to dormant P vivax and P ovale can recur months after the initial infection, even one that has been treated.
Mixed infections involving more than one species of Plasmodium may occur in areas of high endemicity and multiple circulating malarial species. In these cases, clinical differentiation and decision making is important. Relapsing fever intervals correspond with circulating parasitic stages, and, although much may be made of the typical intervals for fever recurrence (quartan, tertiary, quotidian), in reality, variability makes this an unreliable indicator for species case identification. The clinician should have a low threshold for including treatment for P falciparum or P knowlesi infection to avoid incomplete or inadequate treatment of these more dangerous infections.
Although local Plasmodium transmission is rare in the United States (despite recent cases of P vivax transmitted locally), malaria was once widely endemic in the United States, and there is the potential for a resurgence of endemic malaria. It is endemic in Mexico, where it is considered by the WHO to be in the pre-elimination phase. Anopheles mosquito species exist and malaria occurs on all continents but Antarctica. Plasmodium infection should be considered in patients with no history of international travel but who present with an otherwise unexplained fever, chills, aches, anemia, metabolic acidosis, acute respiratory distress syndrome (ARDS), renal failure, central nervous system (CNS) dysfunction, gastrointestinal symptoms, or sepsis.
Vaccines for preventing Malaria have been pursued for decades, however there are many challenges with this development due to the complex immunochemical profile of the Plasmodium parasite. There is no licensed malaria vaccine in production; however, there have been several recent breakthroughs that may change this in the near future. Namely, there are promising prospects in the fields of Sporozoite Subunit Vaccines, Liver-Stage Subunit Vaccines, Blood-Stage Vaccines, Radiation-Attenuated Whole Sporozoite Vaccines, and Surface Protein Pfs Transmission-Blocking Vaccines. [6]
Go to Malaria and Malaria in Children for complete information on these topics.
Emergency Department Care
Assess the patient’s airway, breathing, circulation, and neurologic status and intervene as necessary. A protective airway may be indicated in cases of severe CNS complications. Personal protective equipment and strict fluid precautions should be used upon risk of infectious viral hemorrhagic illness based on the patient history, especially in light of the recent Ebola outbreak, which had a substantial coinfection rate, with consequences in terms of both morbidity and mortality. [7] A malaria-sensitive triage score for Ebola exists, given the overlap in clinical presentation and distribution of the disease, but it was derived and designed for Ebola treatment centers, not emergency department triage in nonendemic areas. [8]
Cerebral malaria often manifests as altered mental status and seizures but may present as coma. Visual disturbances are associated with cerebral malaria and indicate the need for a comprehensive evaluation for other signs and symptoms that may not be initially apparent. [9]
If evidence of life-threatening hemolytic anemia is determined, establish large-bore intravenous (IV) lines, provide fluid resuscitation, and administer transfusion of type-specific packed RBCs.
Evaluate and treat the patient for acute renal failure that may be due to the nephrotoxic products of hemolysis or hypovolemia due to gastrointestinal fluid losses and poor oral intake.
Hyponatremia is probably associated with continued oral hypotonic fluid intake in the setting of hypovolemia and does not require treatment beyond rehydration. [10] Overly aggressive treatment of hyponatremia may lead to death.
Monitor and treat hypoglycemia as needed and search for any signs of microvascular malarial complications.
Laboratory analysis with polymerase chain reaction (PCR) is helpful, although it is not always readily available to determine the Plasmodium species, level of drug resistance, and degree of parasitemia. [11] Obtain a complete history for the laboratory, including the likely country or region of origination. There may be morphological similarity between some species, so microscopic examination is not necessarily determinative.
P knowlesi may be confused with either P falciparum or P malariae at different stages. This is important in that P malariae infection is generally more benign, whereas P falciparum and P knowlesi are more likely to cause severe malaria. It should be noted that P vivax is more common globally and that it may also cause severe malaria. The Centers for Disease Control and Prevention (CDC) should be contacted to assist in risk assessment and testing for Plasmodium and viral hemorrhagic illness, as the two may overlap in presentation and may coexist in a single patient.
If the infection is caused by an unidentified species or by mixed plasmodia species, treat it as if it were caused by P falciparum. In the absence of known drug sensitivities, assume that the Plasmodium species in question is chloroquine resistant. If Southeast Asia is the origin of the infection, then assume mefloquine resistance. Drug resistance is known for malaria caused by P falciparum, P vivax, and P malariae.
It is unlikely that the emergency physician will know the patient’s degree of parasitemia. However, if it is known to be greater than 10% or if the patient is experiencing life-threatening complications (ie, coma, respiratory failure, coagulopathy, fulminant kidney failure), exchange transfusion may be investigated as a treatment option.
Administer parenteral antimalarial therapy to eradicate the protozoa from the bloodstream per CDC and WHO guidelines. The CDC publishes guidelines [12] and a treatment table, [13] and maintains a webpage for the diagnosis and treatment of malaria in the United States. [14] The CDC no longer recommends the use of exchange transfusion for the treatment of severe malaria. [15] In addition, it is recommended to call the CDC Malaria Hotline: (770) 488-7788 or (855) 856-4713 (toll-free) Monday–Friday 9am–5pm EST - (770) 488-7100 after hours, weekends, and holidays not only as a resource to providers, but also as a tool the CDC uses for data collection and monitoring.
Consider human immunodeficiency virus (HIV) testing if indicated. HIV and malaria coinfection is a significant problem across Asia and sub-Saharan Africa, where both diseases may be relatively common. Malaria and HIV coinfection can lead to worse clinical outcomes in both disease processes, with malarial infections being more severe in HIV-infected patients and HIV replication increasing in malaria infection, and transplacental HIV transmission is increased in gravid patients with malaria.
CDC guidelines for treating patients with malaria in the US is determined by infection severity, country of origin of the infection, drug susceptibility (eg, chloroquine resistance), and the Plasmodium species identified. [16]
Artesunate IV was officially approved by the FDA in May 2020 (it was previously available from the CDC through an IND protocol). It is indicated for initial treatment of severe malaria in adults and children. Approval was based the South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) and the African Quinine Artesunate Malaria Trial (AQUAMAT). These 2 studies examined a total of 6,886 patients and included adults, children, and pregnant women. Artesunate IV reduced mortality by 34.7% (p = 0.0002) and 22.5% (p = 0.002). compared with quinine in the SEAQUMAT and AQUAMAT studies respectively. [17, 18]
The WHO also has established guidelines for the treatment of malaria [19] and management of severe malaria. [20] Malarial disease exists on a spectrum, and symptoms vary based on the patient's progression from infection to asymptomatic parasitemia to uncomplicated illness through severe malaria and death. By definition, severe malaria carries a high mortality rate and is predominantly due to P falciparum infection. The WHO updated the classification of severe malaria in 2014 and recommended dividing patients into three subgroups. [21]
Group 1, the most critical patients, are generally treated with parenteral antimalarial medication and require intensive supportive care and resuscitation. Group 1 includes all patients who present with prostration or altered mental status (ie, confusion, agitation, or coma). Furthermore, any patient who presents with extreme acidotic breathing (rapid, deep breathing with retractions), hypotension, anuria, or significant GI bleeding belongs in this group.
Group 2 can usually be treated with oral artemisinin-based combination therapy (ACT) but require close medical observation owing to an increased risk for clinical decompensation. Group 2 consists of patients who do not possess any characteristics of those in group 1 but who present with anemia (hemoglobin < 7 g/dL or hematocrit < 20%), hemoglobinuria, jaundice, or one or more convulsions during the preceding 24-hour period.
Group 3 is the designation reserved for patients who require parenteral antimalarials due to continuing intractable vomiting but do not exhibit any of the features of group 1 or 2.
Diagnostic delay of malaria in non-endemic has been an ongoing problem. Both from a perspective of delay in patient presentation and early identification, as well as a different population immunogenic profiles and chemoprophylaxis use, there are clear challenges when encountering malaria in non-endemic countries.
One point of controversy is treatment of G6PD patients infected with malaria. Currently, adjunctive primaquine is the only therapy capable of killing mature P falciparum mature gametocytes [4] and is the drug of choice in clearing P vivax dormant hypnozoites (dayanda) in relapsing patients who failed initial therapy. Primaquine is known to cause significant hemolysis in G6PD patients, however based on the dose and time course this effect can be significantly reduced. Therefor the WHO indicates that the G6PD status of patients should be used to guide administration of primaquine as a Good Practice Statement, and recommends specific weight based dosing in patients known to have G6PD.
Admission Guidelines
General hospital admission guidelines are as follows:
-
Patients with suspected or confirmed P falciparum or P knowlesi infection
-
Children
-
Pregnant women
-
Immunodeficient individuals
Intensive care unit admission guidelines are as follows:
-
Immediate life-threatening complications present, such as coagulopathy, hypoglycemia, or end-organ failure
-
Presence of signs and symptoms consistent with cerebral malaria (eg, altered mental status, repeated seizures, coma)
-
Patients who are nonimmune with a Pfalciparum parasitemia greater than 2% or who are semi-immune with a P falciparum parasitemia greater than 5%
-
Presence of any other severe malarial complications
Outpatient Considerations
A reliable, immunocompetent, adult patient with mild presentations of P vivax, P ovale, or P malariae infection may be treated on an outpatient basis. However, special care must be taken if P malariae is diagnosed solely based on blood smear, as it may be confused with the sometimes fatal P knowlesi infection that would require inpatient treatment. Persons treated as outpatients should have adequate follow-up care, including daily blood smears to confirm the treatment’s effectiveness in decreasing parasitemia and to evaluate treatment compliance. All cases, regardless of severity at the time of presentation, should be reported to the CDC.
Consultations
It is recommended that the emergency physician contact an infectious disease clinician or pathologist when confronted with a possible case of malaria based on history and physical examination to ensure proper identification and diagnosis. It is particularly recommended that the physician contact the CDC directly for any known or suspected case.
To aid in identification of the species of Plasmodium, also notify the pathologist of the patient’s information, including the following:
-
Where the patient has traveled and when the patient returned home
-
Whether the patient has ever before been diagnosed with malaria, and if known, which species of Plasmodium caused the previous infection
-
What medication or prophylaxis the patient has taken and when the last dose was administered
-
Whether the patient has a history of blood transfusion or of nonsterile needle usage
-
At what date and time the patient's blood sample was drawn and what condition the patient was in at that time (eg, patient was symptomatic, any periodicity of symptoms); also provide an indication of the severity of illness
-
Whether the patient may have had recent contact (past 1-2 months) with an individual who was ill with a febrile illness
The CDC has malaria diagnosis and treatment guidelines for US clinicians and guidelines on the investigation of locally acquired mosquito-transmitted malaria. [22, 23]
-
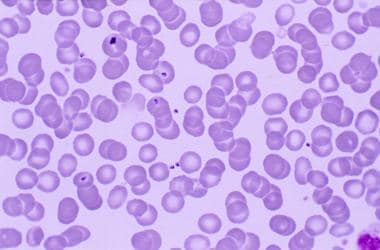
Malarial merozoites in the peripheral blood. Note that several of the merozoites have penetrated the erythrocyte membrane and entered the cell.