Practice Essentials
Abdominal aortic aneurysms (AAAs) are relatively common and are potentially life-threatening. Patients at greatest risk for AAA are men who are older than 65 years and have peripheral atherosclerotic vascular disease. See the image below.
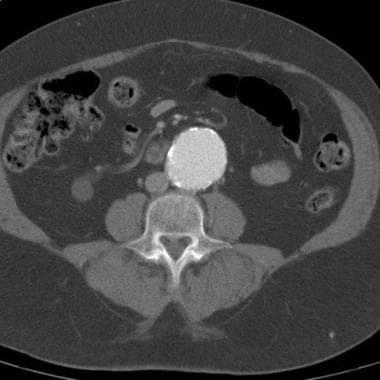 CT demonstrates abdominal aortic aneurysm (AAA). Aneurysm was noted during workup for back pain, and CT was ordered after AAA was identified on radiography. No evidence of rupture is seen.
CT demonstrates abdominal aortic aneurysm (AAA). Aneurysm was noted during workup for back pain, and CT was ordered after AAA was identified on radiography. No evidence of rupture is seen.
Signs and symptoms
AAAs are usually asymptomatic until they expand or rupture. An expanding AAA causes sudden, severe, and constant low back, flank, abdominal, or groin pain. Syncope may be the chief complaint, however, with pain less prominent.
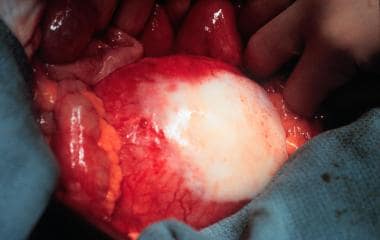
Most clinically significant AAAs are palpable upon routine physical examination. The presence of a pulsatile abdominal mass is virtually diagnostic but is found in fewer than half of all cases.
Patients with a ruptured AAA may present in frank shock, as evidenced by cyanosis, mottling, altered mental status, tachycardia, and hypotension. Whereas abrupt onset of pain due to rupture of an AAA may be quite dramatic, associated physical findings may be very subtle. Patients may have normal vital signs in the presence of a ruptured AAA as a consequence of retroperitoneal containment of hematoma.
At least 65% of patients with a ruptured AAA die of sudden cardiovascular collapse before arriving at a hospital.
See Presentation for more detail.
Diagnosis
No specific laboratory studies can be used to diagnose AAA. The following imaging studies, however, can be employed diagnostically:
-
Ultrasonography - Standard imaging technique for AAA
-
Plain radiography - Using this method to evaluate patients with AAA is difficult because the only marginally specific finding, aortic wall calcification, is seen less than half of the time
-
Computed tomography (CT) and CT angiography (CTA) - This form of imaging is the main modality for defining and planning open or endovascular AAA repair; CT offers certain advantages over ultrasonography in defining aortic size, rostral-caudal extent, involvement of visceral arteries, and extension into the suprarenal aorta
-
Magnetic resonance imaging - This permits imaging of the aorta comparable to that obtained with CT and ultrasonography, without subjecting the patient to dye load or ionizing radiation
-
Angiography - With the fine resolution afforded by CTA, conventional angiography is rarely indicated to define the anatomy
See Workup for more detail.
Management
AAAs are treated with surgical repair. When indicated, unruptured aneurysms can be addressed with elective surgery, whereas ruptured AAAs necessitate emergency repair. The primary methods of AAA repair are as follows:
-
Open - This requires direct access to the aorta via a transperitoneal or retroperitoneal approach
-
Endovascular - This involves gaining access to the lumen of the abdominal aorta, usually via small incisions over the femoral vessels; an endograft, typically a polyester or Gore-Tex graft with a stent exoskeleton, is placed within the lumen of the AAA, extending distally into the iliac arteries
See Treatment and Medication for more detail.
Background
Abdominal aortic aneurysms (AAAs) are relatively common and are potentially life-threatening. Aneurysms are defined as a focal dilatation in an artery, with at least a 50% increase over the vessel’s normal diameter. Thus, enlargement of the diameter of the abdominal aorta to 3 cm or more fits the definition.
AAA usually results from degeneration in the media of the arterial wall, leading to a slow and continuous dilatation of the lumen of the vessel (see Pathophysiology). Uncommon causes include infection, cystic medial necrosis, arteritis, trauma, inherited connective-tissue disorders, and anastomotic disruption. (See Etiology.)
AAAs generally affect elderly white men. Smoking appears to be the risk factor most strongly associated with AAA. In addition to increasing age and male sex, other factors include increased height, weight, body mass index, and body surface area. A familiar clustering has been noted in 15-25% of patients undergoing surgical repair of AAA. Female sex, African American race, and the presence of diabetes mellitus are negatively associated with AAA. [1, 2]
Most AAAs are asymptomatic, and many are detected as an incidental finding on diagnostic imaging obtained for other reasons. There is a wide spectrum of clinical presentations, and AAA should be considered in the differential diagnosis for a number of symptoms.
Ultrasonography is the standard imaging tool for AAA. Bedside emergency ultrasonography should be performed immediately if AAA is suspected. (See Workup and Bedside Ultrasonography Evaluation of Abdominal Aortic Aneurysm.)
Treatment of abdominal aortic aneurysms (AAAs) is with surgical repair. When indicated, unruptured aneurysms can undergo elective repair (see Treatment). The combination of ultrasonographic screening, reduced preoperative risk, and new minimally invasive techniques extend aortic aneurysm treatment into an increasingly elderly population. If an AAA ruptures, emergency surgical repair is required.
For patient education information, see the Circulatory Problems Center and the Cholesterol Center, as well as Aortic Aneurysm, High Cholesterol, and Cholesterol FAQs.
Anatomy
The abdominal aorta has three distinct tissue layers: intima, media, and adventitia. The intima is composed of the classic endothelial layer. The media comprises smooth muscle cells surrounded by elastin, collagen, and proteoglycans; to a great extent, this layer is responsible for the structural and elastic properties of the artery. The adventitia consists primarily of collagen but also contains a variety of cells (including fibroblasts and immunomodulatory cells), as well as adrenergic nerves.
The diameter of the aorta decreases from its thoracic portion to its abdominal and infrarenal portions. A normal aorta shows a reduction in medial elastin layers from the thoracic portion to the abdominal portion. Elastin content and collagen content are also reduced.
Most AAAs begin below the renal arteries and end above the iliac arteries. The size, shape, and extent of AAAs vary considerably. Like aneurysms of the thoracic aorta, AAAs may be broadly described as either fusiform (circumferential) or saccular (more localized). However, these descriptions represent two points on a continuum, and lesions that fall between the two points exist.
The important surgical and endovascular anatomic considerations include associated renal and visceral artery involvement (either occlusive disease or involved in the aneurysm process) and the iliac artery (either occlusive disease or aneurysms). The length of the infrarenal aortic neck is important in helping determine the surgical approach (ie, retroperitoneal or transabdominal) and the location of the aortic cross-clamp.
Consideration of hypogastric artery (internal iliac) outflow is important in planning surgical repair. Loss of blood flow from the hypogastric artery may result in impotence in males and sigmoid colon ischemia with necrosis.
Inflammatory aneurysms represent a subsegment of AAA and are characterized by a thick inflammatory peel. These aneurysms are associated with retroperitoneal fibrosis and adhesion of the duodenum and fibrosis (see the image below).
Pathophysiology
AAAs arise as a result of a failure of the major structural proteins of the aorta (elastin and collagen). The inciting factors are not known, but a genetic predisposition clearly exists. Although aneurysms represent a dilatation in all layers of the vessel wall, AAAs develop after degeneration of the media. The degeneration ultimately may lead to widening of the vessel lumen and loss of structural integrity.
After age 50 years, the normal diameter of the infrarenal aorta is 1.5 cm in women and 1.7 cm in men. An infrarenal aorta that is 3 cm or more in diameter is considered an AAA, even if asymptomatic. Approximately 90% of AAAs are infrarenal.
A multidisciplinary research program supported by the US National Heart, Lung, and Blood Institute identified the following as mechanisms important in the development of AAA [3] :
-
Proteolytic degradation of aortic-wall connective tissue
-
Inflammation and immune responses
-
Biomechanical wall stress
-
Molecular genetics
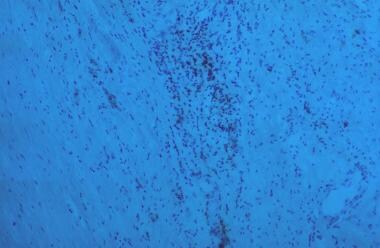
Similarly, surgical specimens of AAA reveal the following (see the image below):
-
Inflammation, with infiltration by lymphocytes and macrophages
-
Thinning of the media
-
Marked loss of elastin
Elastin
Elastin is the principal load-bearing element in the aorta. The aortic wall contains smooth muscle, elastin, and collagen arranged in concentric layers in order to withstand arterial pressure. The number of medial elastin layers is markedly reduced from the proximal thoracic aorta to the infrarenal aorta, with medial thinning and intimal thickening.
Elastin fragmentation and degeneration are observed in aneurysm walls. The decrease in content coupled with the histologic changes of this matrix protein in aneurysms may explain the propensity for aneurysm formation in the infrarenal aorta.
Proteolysis, metalloproteinases, and inflammation
In AAA, the aortic media appears to degrade by way of a proteolytic process. This implies an increase in the concentration of proteolytic enzymes relative to the concentration of their inhibitors in the abdominal aorta as the individual ages.
Some research has focused on the role of the metalloproteinases, a group of zinc-dependent enzymes responsible for tissue remodeling. Reports have documented increased expression and activity of matrix metalloproteinases (MMPs) in people with AAAs. MMPs and other proteases have been shown to be secreted into the extracellular matrix of AAAs by macrophages and aortic smooth-muscle cells.
MMPs and their inhibitors are present in normal aortic tissue and are responsible for vessel-wall remodeling. Aneurysmal tissue tends to demonstrate increased MMP activity and decreased inhibitor activity, which favor the degradation of elastin and collagen. The mechanism that tips the balance in favor of degradation of elastin and collagen in the aortic wall of AAAs by MMPs and other proteases is not yet known.
Upon histologic examination, AAAs demonstrate a chronic adventitial and medial inflammatory infiltrate. Infiltration of AAAs with lymphocytes and macrophages may trigger protease activation via various cytokines (eg, interleukin [IL]-1, IL-6, IL-8, and tumor necrosis factor [TNF]-α).
Immunoreactive proteins are found more conspicuously in the abdominal aorta, and this may contribute to the increased frequency of aneurysms in this location. Further study has defined a matrix protein that is immunoreactive with immunoglobulin G in the aneurysm wall. This autoantigen appears to be a collagen-associated microfibril. Certain infectious agents (eg, Chlamydia pneumoniae and Treponema pallidum) have been associated with the development of this protein; however, no direct cause-and-effect relation has been demonstrated.
Insights from molecular genetics
Through gene microarray analysis, various genes involved in extracellular matrix degradation, inflammation, and other processes observed in AAA formation have been shown to be upregulated, whereas others that may serve to prevent this occurrence are down-regulated. The combination of proteolytic degradation of aortic-wall connective tissue, inflammation and immune responses, biomechanical wall stress, and molecular genetics represents a dynamic process that leads to aneurysmal deterioration of aortic tissue.
Atherosclerosis
Most AAAs occur in individuals with advanced atherosclerosis. Atherosclerosis may induce AAA formation by causing mechanical weakening of the aortic wall with loss of elastic recoil, along with degenerative ischemic changes, through obstruction of the vasa vasorum.
Many patients with advanced atherosclerosis do not develop AAA, and some patients having no evidence of atherosclerosis do. The observed association between atherosclerosis and AAA probably is not causative; however, atherosclerosis may represent a nonspecific secondary response to vessel-wall injury that is induced by multiple factors.
Etiology
AAA is thought to be a degenerative process of the aorta, the cause of which remains unclear. It is often attributed to atherosclerosis because these changes are observed in the aneurysm at the time of surgery. However, a study by Blanchard et al found that the risk factors for AAA differ from those for atherosclerosis, with no association between cholesterol and AAA. [1] In addition, atherosclerosis fails to explain the development of occlusion, which is observed in the disease process.
Patients at greatest risk for AAA are men who are older than 65 years and have peripheral atherosclerotic vascular disease. A history of smoking often is elicited. Accordingly, in 2005, the US Preventive Services Task Force (USPSTF) recommended screening with ultrasonography in men aged 65-75 years who had ever smoked. [4] These recommendations were subsequently updated [5] on the basis of evidence from a 2014 study by Guirguis-Blake et al. [6]
A Swedish study showed that instances of AAA in elderly men have been decreasing, A phenomenon that can be attributed to a nationwide decline in smoking for the past 30 years, as well as the significantly improved longevity of the elderly population. [7] A one-time ultrasound scan is recommended for men once they reach age 65 years to detect and prevent possible occurrences of AAA.
Other risk factors for AAA include the following:
-
Chronic obstructive pulmonary disease (COPD)
-
Previous aneurysm repair or peripheral aneurysm (popliteal or femoral)
-
Hypertension (1-15% of cases)
Less frequent causes of AAA include Marfan syndrome, Ehlers-Danlos syndrome, and collagen-vascular diseases. In fewer than 5% of cases, AAA is caused by mycotic aneurysm of hematogenous origin. In these cases, local invasion of the intima and media gives rise to abscess formation and aneurysmal dilation of the vessel. Gram-positive organisms most commonly cause mycotic aneurysms. Other uncommon causes include cystic medial necrosis, arteritis, trauma, and anastomotic disruption producing pseudoaneurysms.
Persons who have first-degree relatives with AAA are at increased risk for AAA. The familial prevalence rate of AAA has been estimated at 15-25%. Studies by Majumder et al suggest that the genetic predisposition is isolated to a single dominant gene with low penetrance that increases with age. [8]
Tilson et al described the potential for an autoimmune basis for the development of AAA involving the DRB1 major histocompatibility locus. [9] This locus has been identified as a basis for inflammatory AAA.
In late 2018, the FDA issued a warning that fluoroquinolone use can increase the risk of aortic aneurysm and urged healthcare providers to avoid prescribing these antibiotics to patients with or at risk for an AA, such as those with peripheral atherosclerotic vascular disease, hypertension, or certain genetic conditions (eg, Marfan syndrome and Ehlers-Danlos syndrome), as well as the elderly. [10]
Risk factors for rupture
Aneurysm diameter is an important risk factor for rupture. In general, AAAs gradually enlarge (0.2-0.8 mm/year) and eventually rupture. Hemodynamic factors play an important role. Areas of high stress have been found in AAAs and appear to correlate with the site of rupture. Computer-generated geometric models have demonstrated that aneurysm volume is a better predictor of areas of peak wall stress than aneurysm diameter. This may have implications for determining which AAAs require surgical repair.
AAA rupture is believed to occur when the mechanical stress acting on the wall exceeds the strength of the wall tissue. Wall tension can be calculated by applying Laplace’s law, as follows:
-
P × R/W
where P is the mean arterial pressure (MAP), R is the radius of the vessel, and W is the thickness of the vessel wall. AAA wall tension is a significant predictor of pending rupture. The actual tension in the AAA wall appears to be a more sensitive predictor of rupture than aneurysm diameter alone. For these reasons, the clinician may wish to achieve acute blood pressure control in patients with AAA and elevated blood pressure.
Epidemiology
United States statistics
In autopsy studies, the frequency rate of AAA ranges from 0.5% to 3.2%. In a large US Veterans Affairs screening study, the prevalence was 1.4%. [2] The likelihood of development ranges from 3 to 117 cases per 100,000 person-years.
Ruptured AAA is the 13th-leading cause of death in the United States, causing an estimated 15,000 deaths per year. The frequency of rupture is 4.4 cases per 100,000 persons. The reported incidence of rupture ranges from 1 to 21 cases per 100,000 person-years. Despite increased survival following diagnosis, incidence and crude mortality seem to be increasing.
International statistics
The frequency rate of asymptomatic AAA is 8.2% in the United Kingdom, 8.8% in Italy, 4.2% in Denmark, and 8.5% in Sweden (in males only). The frequency rate of AAA in females is much lower, 0.6-1.4%. The frequency of AAA rupture is 6.9 cases per 100,000 persons in Sweden, 4.8 cases per 100,000 persons in Finland, and 13 cases per 100,000 persons in the United Kingdom.
Age-, sex-, and race-related demographics
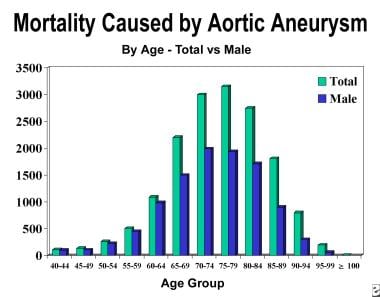
The incidence of AAA begins to increase sharply after 50 years of age and peaks in the eighth decade of life (see the image below). In women, the onset is delayed and appears to begin at approximately age 60 years.
The male-to-female incidence ratio in people younger than 80 years is 2:1. In those older than 80 years, the ratio is 1:1. White men have the highest incidence of AAA (~3.5 times that in African American men). AAAs are uncommon in African Americans, Asians, and persons of Hispanic heritage.
Prognosis
For patients who suffer rupture of an AAA before hospital arrival, the prognosis is guarded. More than 50% do not survive to reach the emergency department; for those who do, the survival rate drops by about 1% per minute. However, in the subset of patients who are not in severe shock and who receive timely, expert surgical intervention, the survival rate is good.
In 1988, 40,000 surgical reconstructions for AAA were performed in the United States, and substantial mortality differences between elective and emergency operations were noted. Because the mortality associated with elective aneurysm repair is drastically lower than that associated with repair of a ruptured AAA, the emphasis must be on early detection and repair free from complications.
In 2014, Ambler et al, in association with the Audit and Quality Improvement Committee of the Vascular Society of Great Britain and Ireland, used data collected during a 15-month period in the United Kingdom National Vascular Database to develop a model for assessing the risk of in-hospital mortality after AAA repair. [11]
The long-term prognosis is related to associated comorbidities. Long-term survival is shortened by chronic heart failure and COPD. Rupture of associated thoracic aneurysms is also an important cause of late death. Overall, AAA repair is very durable, with few long-term complications (< 5% false aneurysm). In general, the survival rate of patients with successful AAA repair is comparable to that of people in the age-matched population at large who have never had an aneurysm. [12, 13]
-
Radiograph shows calcification of abdominal aorta. Left wall is clearly depicted and appears aneurysmal; however, right wall overlies spine.
-
On radiography, lateral view clearly shows calcification of both walls of abdominal aortic aneurysm, allowing diagnosis to be made with certainty.
-
CT demonstrates abdominal aortic aneurysm (AAA). Aneurysm was noted during workup for back pain, and CT was ordered after AAA was identified on radiography. No evidence of rupture is seen.
-
Arteriography demonstrates infrarenal abdominal aortic aneurysm. This arteriogram was obtained in preparation for endovascular repair of aneurysm.
-
Lateral arteriogram demonstrates infrarenal abdominal aortic aneurysm. Demonstration of superior mesenteric artery, inferior mesenteric artery, and celiac artery on lateral arteriogram is important for complete evaluation of extent of aneurysm.
-
Arteriogram after successful endovascular repair of abdominal aortic aneurysm.
-
Ultrasonogram from patient with abdominal aortic aneurysm (AAA). This aneurysm was best visualized on transverse or axial image. Patient underwent conventional AAA repair.
-
MRI of 77-year-old man with leg pain believed to be secondary to degenerative disk disease. During evaluation, abdominal aortic aneurysm was discovered.
-
Age is risk factor for development of aneurysm.
-
Inflammation, thinning of media, and marked loss of elastin.
-
Pulsatile abdominal mass.
-
Aneurysm with retroperitoneal fibrosis and adhesion of duodenum.
-
Aortic endoprosthesis (Cook aortic and aortobi-iliac endograft).
-
Endoaneurysmorrhaphy
-
Endovascular grafts.
-
Atheroemboli from small abdominal aortic aneurysms produce livedo reticularis of feet (ie, blue toe syndrome).
-
Enhanced spiral CT scans with multiplanar reconstruction and CT angiogram.
-
Angiography is used to diagnose renal area. In this instance, endoleak represented continued pressurization of sac.