Background
Giardiasis is a major diarrheal disease found throughout the world. The flagellate protozoan Giardia intestinalis (previously known as G lamblia or G duodenalis), its causative agent, is the most commonly identified intestinal parasite in the United States [1, 2] and the most common protozoal intestinal parasite isolated worldwide. [3, 4, 5, 6, 7] Infection is more common in children than in adults. [8, 9]
G intestinalis can cause asymptomatic colonization or acute or chronic diarrheal illness. The organism has been found in as many as 80% of raw water supplies from lakes, streams, and ponds and in as many as 15% of filtered water samples. [10, 11] It is a common cause of chronic diarrhea and growth retardation in children in developing countries.
Giardiasis usually represents a zoonosis with cross-infectivity between animals and humans. Giardia intestinalis has been isolated from the stools of beavers, dogs, cats, and primates. Beavers may be an important reservoir host for G intestinalis. [12, 13, 14] Other Giardia species include G muris in rodents; G agilis in amphibians; G psittaci and G ardeae in birds; and G microti in voles and muskrats. [15, 16, 17]
Giardia species are endemic in areas of the world that have poor sanitation. In developing countries, the disease is an important cause of morbidity. Water-borne and food-borne outbreaks are common. Because ingestion of as few as 10 Giardia cysts may be sufficient to cause infection, giardiasis is common in daycare center attendees and institutionalized patients in developed countries. G intestinalis is a particularly significant pathogen for people with malnutrition, immunodeficiencies, or cystic fibrosis.
High-risk groups for giardiasis include travelers to highly endemic areas, immunocompromised individuals, and sexually active homosexual men. Cyst passage rates as high as 20% have been reported among certain groups of sexually active homosexual men; these individuals were frequently symptomatic. [12, 18]
The traditional basis of diagnosis is identification of Giardia intestinalis trophozoites or cysts in the stool of infected patients via a stool ova and parasite (O&P) examination. Stool antigen enzyme-linked immunosorbent assays also are available. (See Workup.) Standard treatment for giardiasis consists of antibiotic therapy. Metronidazole, tinidazole, and nitazoxanide are the drugs of choice. Metronidazole is the most commonly prescribed antibiotic for this condition; tinidazole is considered a first-line agent outside the United States. [19] (See Treatment& and Medication.)
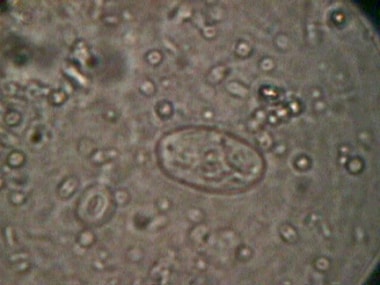
A G intestinalis cyst is seen in the image below.
See What's Eating You: 12 Common Intestinal Parasites and 10 Cases of Food Poisoning: Find the Pathogen Responsible, Critical Images slideshows, to help make an accurate diagnosis.
Historical background
Giardia was originally observed by von Leeuwenhoek in 1681, in his own diarrheal stool, and was described by Vilem Dusan Lambl in 1859 and by Alfred Giard in 1895. The organism's previous name, honoring the contributions of Giard and Lambl, was bestowed in 1915.
Although G intestinalis was the first protozoan parasite described, its role as a pathogenic organism was not recognized until the 1970s, after community outbreaks and after the appearance of the disease in travelers returning from endemic regions. Prior to that time, the organism was thought to be a harmless commensal organism of the intestine. [20]
Pathophysiology
Infection with Giardia intestinalis most often results from fecal-oral transmission or ingestion of contaminated water. Contaminated food is a less common etiology. Person-to-person spread is common, with 30% of family members with infected children themselves becoming infected. [21, 22]
Most infections are asymptomatic. The rate of symptomatic infection in the natural setting varies from 5% to 70%. Giardia is found in healthy people in endemic areas, and asymptomatic carriage with excretion of high numbers of cysts in stools is common. [22, 23]
Predisposing factors to symptomatic infection include hypochlorhydria, various immune system deficiencies, blood group A, and malnutrition. The incubation period averages 1-2 weeks, with a mean of 9 days. The average duration of symptoms in all ages ranges from 3 to 10 weeks. [21]
Giardia life cycle
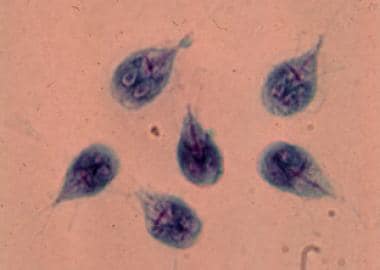
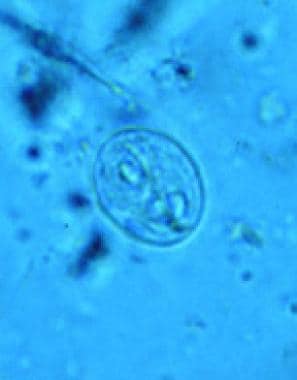
Giardia has one of the simplest life cycles of all human parasites. The life cycle is composed of two stages: (1) the trophozoite (see the first image below), which exists freely in the human small intestine; and (2) the cyst (see the second image below), which is passed into the environment. No intermediate hosts are required.
Upon ingestion of the cyst, contained in contaminated water or food, excystation occurs in the stomach and the duodenum in the presence of acid and pancreatic enzymes. The trophozoites pass into the small bowel where they multiply rapidly, with a doubling time of 9-12 hours. As trophozoites pass into the large bowel, encystation occurs in the presence of neutral pH and secondary bile salts. Cysts are passed into the environment, and the cycle is repeated.
The trophozoite form of G lamblia is teardrop-shaped and measures 9-21 micrometers long by 5-15 micrometers wide. The trophozoite has a convex dorsal surface and a flat ventral surface that contains the ventral disk, a rigid cytoskeleton composed of microtubules and microribbons. The trophozoite also contains four pairs of flagella, directed posteriorly, that aid the parasite in moving. Two symmetric nuclei with prominent karyosomes produce the characteristic facelike image that appears on stained preparations.
The ventral disk, which is often referred to as the sucking or adhesive disk, provides the parasite with powerful adhesion, catching, and holding abilities. In the murine model of giardiasis, the ventral disk adhesion imprints are marked but less impressive than in the human small intestine. However, this direct injury is an unlikely cause of the more extensive reduction in microvillus surface area, the reduction in disaccharidase activities, and the more pronounced abnormalities of villous architecture that are seen in giardiasis. [24, 25]
The cyst form of the protozoan is smooth-walled and oval in shape, measuring 8-12 micrometers long by 7-10 micrometers wide. As the cyst matures, nuclear division occurs and readies the cyst to release two trophozoites upon excystation. Once the host is infected, trophozoites may appear in the duodenum within minutes. [26] Excystation occurs within 5 minutes of exposure of the cysts to an environment with a pH between 1.3 and 2.7.
After infection, the trophozoites attach to the enterocytes via the ventral adhesive disk. This may occur through the presence of lectin on the surface of the trophozoite or through other mechanical means. Encystation is a continuous process during infection.
As the trophozoites encounter neutral pH and/or secondary bile salts, encystation-specific secretory vesicles (ESVs) appear. After 15 hours, cyst wall proteins are visible. Within 24 hours after the appearance of ESVs, the trophozoite is covered with these cyst wall proteins, the form of the cyst has emerged, and new antigenic differences are present.
Mechanism of injury
The mechanisms by which Giardia causes diarrhea and intestinal malabsorption are probably multifactorial and not yet fully elucidated. [24] Postulated mechanisms include damage to the endothelial brush border, enterotoxins, immunologic reactions, and altered gut motility and fluid hypersecretion via increased adenylate cyclase activity.
Adhesion of trophozoites to the epithelium has been demonstrated to cause increased epithelial permeability. Giardia- induced loss of intestinal brush border surface area, villus flattening, inhibition of disaccharidase activities, and eventual overgrowth of enteric bacterial flora appear to be involved in the pathophysiology of giardiasis but have yet to be causatively linked to the disease's clinical manifestations.
Marked or moderate partial villous atrophy in the duodenum and jejunum can be observed in histologic sections from asymptomatic individuals who are infected. In addition to disrupting the mucosal epithelium, effects in the intestinal lumen may contribute to malabsorption and the production of diarrhea. [8, 27] Nevertheless, diarrhea can still occur in individuals in the absence of obvious light microscopic changes in small intestinal structure.
Varying degrees of malabsorption of sugars (eg, xylose, disaccharides), fats, and fat-soluble vitamins (eg, vitamins A and E) may contribute to substantial weight loss. The histopathologic response to giardiasis varies and does not strongly correlate with clinical symptoms. [13, 28]
G intestinalis may release cytopathic substances that damage the intestinal epithelium. Giardia species contain thiol-dependent and thiol-independent proteinases, which may find substrates in the microvillus membrane. A 2018 report suggests that three main cysteine proteases (CP14019, CP16160 and CP16779) secreted by G intestinalis disrupt intestinal epithelial cell junctional complexes and degrade chemokines. [29] In addition, the surface mannose-binding lectin of G intestinalis may contribute to epithelial damage. Whatever the mechanism by which G intestinalis damages villous epithelial cells, the result consistently appears to be an increase in crypt length and crypt cell proliferation. [30]
Enterocytic injury is mediated by activated host T lymphocytes. Pathophysiological activation of lymphocytes is secondary to Giardia-induced disruption of epithelial tight junctions, which, in turn, increases intestinal permeability. Loss of epithelial barrier function is a result of Giardia-induced enterocyte apoptosis. [27, 31, 32]
Epithelial barrier dysfunction in cases with chronic giardiasis is associated with increased rates of enterocyte apoptosis. Consistent with these observations, microarray analyses of the effects of G intestinalis on human CaCo2 cells found that the parasite–host interactions lead to a pronounced up-regulation of genes implicated in the apoptotic cascade and the formation of reactive oxygen species.
Panaro et al demonstrated that Giardia trophozoites induce cell apoptosis by activation of both intrinsic and extrinsic apoptotic pathways, down-regulation of the antiapoptotic protein Bcl-2, and up-regulation of the proapoptotic Bax. These findings suggest a possible role for caspase-dependent apoptosis in the pathogenesis of giardiasis. [33]
Giardia can also prevent the formation of nitric oxide, a compound known to inhibit giardial growth, by consuming local arginine, which effectively removes the substrate needed by enterocytes to produce nitric oxide. This mechanism may contribute to Giardia-induced enterocyte apoptosis, because arginine starvation in these cells is known to result in programmed cell death. [24, 34]
G intestinalis is genetically heterogeneous with eight genetically distinct genotypes or assemblages, designated A-H; assemblages A and B can infect humans. Genotypes vary within group A and B, which could explain why the role of animals in the epidemiology of human infection remains poorly understood. [35, 36] Some strains appear more biologically suitable than other strains. This feature is potentially important in giardiasis pathogenesis. [37, 38] Genotypically diverse isolates of Giardia species may vary in their ability to produce morphologic changes in the small intestine epithelium and to impair fluid, electrolyte, and solute transport. [39]
Etiology
Giardiasis is caused by the flagellate protozoan Giardia intestinalis (formerly known as G lamblia or G duodenalis). Infection is transmitted through ingestion of infectious G lamblia cysts. [40] The organism is known to have multiple strains with varying abilities to cause disease, and several different strains may be found in one host during infection. The infective dose is low in humans: 10-25 cysts are capable of causing clinical disease in 8 of 25 subjects. Ingestion of more than 25 cysts results in a 100% infection rate. [41]
Person-to-person transmission, often associated with poor hygiene and sanitation, is a primary means of infection. Diaper changing and inadequate hand washing are risk factors for transmission from infected children. Children attending day care centers, as well as day-care workers, have a higher risk of infection secondary to fecal-oral transmission.
Water-borne transmission is responsible for a significant number of epidemics in the United States, generally following ingestion of unfiltered surface water. Giardia cysts retain viability in cold water for as long as 2-3 months. Giardia was implicated in 242 outbreaks (41,000 cases) in the United States from 1971 to 2011 (74.8% waterborne), [42] and 111 outbreaks (760 cases) from 2012 to 2017. [43]
Venereal transmission occurs through fecal-oral contamination. Food-borne epidemics have been reported, most commonly secondary to contamination by infected food-handlers. [44, 45] Pets frequently harbor Giardia in their GI tracts, but they are not thought to be a significant cause of outbreaks in humans.
Epidemiology
United States statistics
Giardia remains the parasite most commonly identified in stool specimens, causing about 1.2 million annual episodes of illness. [2] From 1964 to 1984, G lamblia caused at least 90 water-borne outbreaks of diarrhea, affecting more than 23,000 people. More recently, between 2012 to 2017 there were 111 outbreaks. [43] These outbreaks typically involved small water systems using untreated or inadequately treated surface water.
Most water-borne outbreaks in the United States have occurred in western mountain regions (eg, Rocky Mountains, Sierra Nevada, Cascades) where giardiasis is considered endemic. The incidence of giardiasis is high among individuals who camp and backpack in mountainous Western states. Other groups at increased risk for infection include children, homosexual men, and individuals with immunoglobulin deficiency states (inherited or acquired).
Yoder et al reported that the incidence is greatest in northern states, [46] but this may be related to the differences in individual state surveillance systems and may not necessarily reflect an actual higher incidence. [46] Because water-borne giardiasis outbreaks have been reported in every region in the United States, the diagnosis must be considered anywhere in the country.
Endemic infection occurs most commonly from July through October among children younger than 5 years and adults aged 25-39 years. Carrier rates as high as 30-60% have been documented among children in day care centers, institutions, and on Native American reservations. [18, 47]
The asymptomatic carriage rate in children may be as high as 20% in southern regions and in children younger than 36 months who attend daycare centers. Asymptomatic carriage may persist for several months. Many children with giardiasis who are symptomatic have been shown to spread the disease within their homes, and they may contribute to high endemic rates in their communities. [18]
In the 46 states reporting giardiasis, the mean number of cases per 100,000 population varies by state, with a range of 0.1-23.5 cases. Most cases are reported between June and October and are associated with the summer recreational water season and camping. [48]
International statistics
Giardia has a worldwide distribution, occurring in both temperate and tropical regions. It continues to be the most frequently identified human protozoal enteropathogen. Prevalence rates vary from 4% to 42%. In the industrialized world, overall prevalence rates are 2-5%. In the developing world, G intestinalis infects infants early in life and is a major cause of epidemic childhood diarrhea. Prevalence rates of 15-20% in children younger than 10 years are common. [37, 49] A single study of elementary school children in Ethiopia found a prevalence rate as high as 27.1%. [50]
Giardia is the most common gut parasite in the United Kingdom, and infection rates are especially high in Eastern Europe. Prevalence rates of 0.94-4.66% and 2.41-10.99% have been reported in Italy. [51]
A 2005 study demonstrated a Giardia infection rate of 19.6 per 100,000 population per year in Canada. [52] While the yearly incidence of the disease was stable, a significant seasonal variation was observed, with a peak in late summer to early fall, which correlates with the pattern found in the United States. [52] New Zealand reports more than 30 cases of giardiasis per 100,000 population every year, which is one of the highest among the industrialized countries. [53]
Giardiasis accounts for a relatively small percentage of traveler's diarrhea. It is more likely to be found as the cause of diarrhea that occurs or persists after returning home from travel to developing regions of the world due to its relatively long incubation period and persistent symptoms. Giardia has been identified as the causative agent in a large percentage of cases among travelers to the region of St. Petersburg, Russia, where tap water is the primary source. [54]
The highest prevalence of G intestinalis reached 73.4% in Western Nepal. In Bangladesh, a disparity between health prevention and health spending is observed. The Dhaka study performed within the urban areas had identified G intestinalis in 11% of diarrheal stool specimens. [55]
In Ethiopia, the prevalence has been reported to range from 2.0% to 11.4%. [56] Prevalence of G intestinalis has been reported 13.9% in Côte d'Ivoire. [57]
Race-, sex-, and age-related differences in incidence
Giardiasis does not have any race predilection. Native American populations residing on reservations can have high carrier rates.
Giardiasis is slightly more common in males than in females. [52] A Canadian population study demonstrated infection rates of 21.2 per 100,000 per year versus 17.9 per 100,000 per year for males and females, respectively. [52]
Giardiasis affects people of all ages. Infection is rare during the first 6 months of life in breastfed infants, but infants and young children have an increased susceptibility to giardiasis. Age-specific prevalence of giardiasis continues to rise through infancy and childhood and begins to decline only in adolescence. [18, 58]
Children are particularly at risk for infection through exposure at day-care centers. Many of the epidemics documented over the last 2 decades have originated in day-care centers. Estimates of the prevalence of infection, defined by the presence of cyst passage, have been as high as 20-25% in children younger than 3 years. [59, 60]
According to 2003–2005 data from the Centers for Disease Control and Prevention, the greatest number of reported cases occurred among children aged 1-4 and 5-9 years and adults aged 35-44 years. [46]
Prognosis
The prognosis for patients with giardiasis is often good. Most patients are asymptomatic, and most infections are self-limited. Giardiasis is not associated with mortality except in rare cases of extreme dehydration, primarily in infants or malnourished children.
Several antibiotic agents are available with acceptable efficacy rates to shorten the disease course, although drug resistance has been observed in clinical experience. Untreated, symptomatic giardiasis can last for weeks.
When the parasite persists in the stool, reinfection is possible. [8, 31]
Weight loss, disaccharidase deficiency, malabsorption, and growth retardation are possible complications. [61, 62] G intestinalis has been implicated as the chief cause of growth retardation in infected children, even after other diarrhea-causing agents are controlled. [18, 31]
Some patients may experience persistent symptoms (eg, chronic diarrhea/steatorrhea, malabsorption) despite apparently effective antibiotic treatment, although these usually subside over weeks to months. [26, 48, 58] However, Hanevik et al found symptoms consistent with irritable bowel syndrome (IBS) and/or functional dyspepsia in 76 of 82 patients at least 6 months after eradication of Giardia infection. [63] Patients reported bloating, diarrhea, and abdominal pain, which were exacerbated by specific foods or by physical or mental stress. Another study by Hanevik and colleagues associated giardiasis with the presence of IBS and chronic fatigue even 6 years after infection. [64]
Similarly a longitudinal cohort study (2006-2010) of data from the MarketScan commercial database that evaluated the relationship between a diagnosis of giardiasis and that of IBS found that, even accounting for confounding factors, patients with giardiasis had a greater 1-year incidence of IBS than those without giardiasis. [1] The study included a matched cohort of 3935 patients with giardiasis and 19,663 without giardiasis.
Complications
Complications of giardiasis may include the following:
Patient Education
Patients and at-risk individuals should be instructed regarding appropriate hygiene methods and signs/symptoms of infection. Emphasis should be placed on measures such as careful hand washing after changing diapers. Day-care workers should use meticulous hygiene and careful hand washing to reduce spread between children and to staff. Personal hygiene education to minimize person-to-person transmission in high-risk settings such as residential institutions is helpful.
Patients should be advised that oral-anal and oral-genital contact increase the risk of venereal transmission.
Hikers and travelers to areas where the disease is endemic should be educated. Before drinking surface water, they should disinfect it by boiling or the use of halogenating compounds (eg, chlorine) or filtration devices.
-
Giardiasis. Giardia lamblia, cyst form.
-
Giardiasis. Giardia lamblia trophozoites in culture.
-
Giardiasis. A Giardia intestinalis cyst.
-
Giardiasis. Giardia intestinalis trophozoites on stool examination from a patient with diarrhea.
-
Giardiasis. Giardia trophozoite.
-
Giardiasis. Giardia cyst.