Background
C diphtheria is responsible for both endemic and epidemic diseases, and it was first described in the 5th century BC by Hippocrates. Diphtheria manifests as either an upper respiratory tract or cutaneous infection and is caused by the aerobic gram-positive bacteria, Corynebacterium diphtheria. The infection usually occurs in the spring or winter months. It is communicable for 2-6 weeks without antibiotic treatment. [1, 2, 3] People who are most susceptible to infection are those who are not completely immunized or have low antitoxin antibody levels and have been exposed to a carrier or diseased individual. A carrier is someone whose cultures are positive for the diphtheria species but does not exhibit signs and symptoms. As the number of asymptomatic carriers decrease, the number of diphtheria cases consequently decline. [2, 4]
C diphtheria is a nonencapsulated, nonmotile, gram-positive bacillus (below). Pathogenic strains can result in severe localized upper respiratory infection, localized cutaneous infections, and rarely systemic infection. [1]
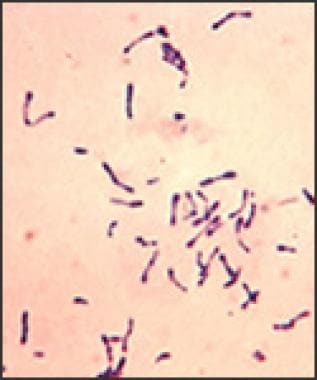 Photomicrograph depicts a number of gram-positive Corynebacterium diphtheriae bacteria, which had been stained using the methylene blue technique. The specimen was taken from a Pai's slant culture.
Photomicrograph depicts a number of gram-positive Corynebacterium diphtheriae bacteria, which had been stained using the methylene blue technique. The specimen was taken from a Pai's slant culture.
Exotoxins are associated with both invasive localized and systemic forms of this disease; however, case reports of invasive disease in absence of the exotoxin release have been documented. [2] Exotoxins are encoded in viral bacteriophages, which are transmitted from bacteria to bacteria. The 3 isolated strains of C diphtheria include gravis, intermedius, and mitis. Intermedius is thought to be responsible for systemic elaboration of the disease, as it is most often associated with the exotoxin. However, all 3 strains can produce toxins. [2, 3]
Corynebacterium ulcerans is a relatively rare species that more frequently causes cutaneous diphtheria; however, this species may rarely cause respiratory symptoms. Disease severity is dependent on exotoxin production. C ulcerans also has been linked to zoonotic transmission to humans and most frequently has been seen in agricultural communities associated with livestock. [5, 6]
The use of diphtheria and tetanus toxoids and acellular pertussis (DTaP) in the United States has greatly decreased the incidence of diphtheria. Although childhood DTaP coverage exceeds 80%, acquired immunity wanes over time, requiring a booster to preserve immunity. Although vaccination is not guaranteed to prevent diphtheria, vaccinated persons who later develop diphtheria have been reported as having milder and fewer fatal infections. [7]
Pathophysiology
Overcrowding, poor health, substandard living conditions, incomplete immunization, and immunocompromised states facilitate susceptibility to diphtheria and are risk factors for transmission of this disease. [8] Human carriers are the main reservoir of infection; however, case reports have linked diphtheria to livestock. [6, 5] Infected patients and asymptomatic carriers can transmit C diphtheria via respiratory droplets, nasopharyngeal secretions, and rarely fomites. [2, 3] In cutaneous disease, contact with wound exudates may result in the transmission of the disease to the skin as well the respiratory tract. [5]
Immunity from exposure or vaccination wanes over time. Inadequate boosting of previously vaccinated individuals may result in increased risk of acquiring the disease from a carrier, even if adequately immunized previously. Additionally, since the advent of widespread vaccination, cases of nontoxigenic strains causing invasive disease have increased. [9]
C diphtheria adheres to mucosal epithelial cells where the exotoxin, released by endosomes, causes a localized inflammatory reaction followed by tissue destruction and necrosis. The toxin is made of 2 joined proteins. [3] The B fragment binds to a receptor on the surface of the susceptible host cell, which proteolytically cleaves the membrane lipid layer enabling segment A to enter. [2] Molecularly, it is suggested that the cellular susceptibility also is due to diphthamide modification, dependent on human leukocyte antigen (HLA) types predisposing to more severe infection. The diphthamide molecule is present in all eukaryotic organisms and is located on a histidine residue of the translation elongation factor 2 (eEF2). eEF2 is responsible for the modification of this histidine residue and is the target for the diphtheria toxin (DT).
Fragment A inhibits an amino acid transfer from RNA translocase to the ribosomal amino acid chain, thus inhibiting protein synthesis required for normal host cell functioning. [2] DT causes a catalytic transfer of NAD to diphthamide, which inactivates the elongation factor, resulting in the inactivation eEF2, which results in protein synthesis blockage and subsequent cell death. [10, 3]
Local tissue destruction enables the toxin to be carried lymphatically and hematologically to other parts of the body. Elaboration of the diphtheria toxin may affect distant organs such as the myocardium, kidneys, and nervous system. Nontoxigenic strains tend to produce less severe infections; however, since widespread vaccination, case reports of nontoxigenic strains of C diphtheria causing invasive disease have been documented. [2]
Transmission
Transmission usually occurs person-to-person through respiratory droplets; diphtheria may also be spread by exposure to infected skin lesions or articles soiled with fluids from these lesions. [11]
On April 18, 2024, the World Health Organization (WHO) published a global technical consultation report that introduced updated terminology for pathogens that are transmitted through the air. [12] These include those that cause COVID-19, influenza, measles, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and tuberculosis.
During 2021-2023, the WHO collaborated with experts from various disciplines and specialties as well as Africa Centres for Disease Control and Prevention; Chinese Center for Disease Control and Prevention; European Centre for Disease Prevention and Control; and United States Centers for Disease Control and Prevention.
Their work “addressed a lack of common terminology” regarding transmission of pathogens through the air that highlighted issues associated with the term “airborne transmission” during the COVID-19 pandemic.
The groups reached consensus on the term ‘infectious respiratory particles,’ which describes pathogens of various particle sizes that can be spread through the air at short and long ranges. “Transmission through the air” may be used as an umbrella term to describe spread of IRPs through the air by either airborne transmission or direct deposition modes.
Epidemiology
Frequency
United States
Since the introduction and widespread use of diphtheria toxoid in the 1920s, respiratory diphtheria has been well controlled, with an incidence of approximately 1000 cases reported annually. Before vaccination, at least 200,000 cases occurred annually in the United States. [13]
Diphtheria remained endemic in some states through the 1970s, with reported incidence rates of greater than 1.0 per million population in Alaska, Arizona, Montana, New Mexico, South Dakota, and Washington. [2] Most of these infections were attributed to incomplete vaccination.
In the United States, diphtheria currently occurs sporadically, mostly among the Native American population, homeless people, lower socioeconomic groups, and alcoholics. [8] Immigrants and travelers from regions with ongoing epidemics are also at risk. [8] The most recent data from the CDC show that, between 1996 and 2018, only 14 cases were reported. [14]
International
According to the World Health Organization (WHO), diphtheria epidemics remain a health threat in developing nations. [3] The largest epidemic recorded since widespread implementation of vaccine programs was in 1990-1995, when a diphtheria epidemic emerged in the Russian Federation, rapidly spreading to involve all Newly Independent States (NIS) and Baltic States. This epidemic caused more than 157,000 cases and 5000 deaths according to WHO reports. [15, 16] Disproportionately high death rates were observed in individuals older than 40 years, and 5,000 deaths were reported. This epidemic accounted for 80% of cases reported worldwide during this time. [17]
From 1993-2003, a decade long epidemic in Latvia resulted in 1359 reported cases of diphtheria with 101 deaths. The incidence fell from 3.9 cases per 100,000 cases in 2001 to 1.12 cases per 100,000 population in 2003. Most cases were registered in unvaccinated adults.
From 1995-2002, 17 cases of cutaneous diphtheria due to toxigenic strains were reported in the United Kingdom. [17]
Overall rates of infection have decreased in Europe from 2000 to 2009, according to the Diphtheria Surveillance Network. This has been attributed to improved vaccination rates creating herd immunity. However, issues with vaccinations still occur, especially in eastern European countries and Russia, and are thought to contribute to the ongoing outbreaks. [18]
Many case reports in the literature describe epidemics in sub-Saharan Africa, France, India, and the United States. [19, 20]
Mortality/Morbidity
Before the introduction of vaccine in the 1920s, the incidence of respiratory disease was 100-200 cases per 100,000 population in the United States and has decreased to approximately 0.001 cases per 100,000 population. [2, 13]
The most widely quoted diphtheria mortality rate is 5-10%. It may reach higher than 20% in children younger than 5 years and adults older than 40 years. Immunization patterns have the most influence on mortality patterns. Mortality rates have not changed significantly over the past few decades. Most deaths occur on days 3-4 secondary to asphyxia with a pharyngeal membrane or due to myocarditis. Mortality rates of 30-40% have been reported for bacteremic disease. [13]
Race
No racial predilection for diphtheria has been reported.
Sex
No significant differences exist between the incidence of diphtheria in males and females. In certain regions of the world, however, women may have lower immunization rates than males. Female infants and young children account for most deaths in endemic regions.
Age
Historically, diphtheria has been primarily a disease of childhood, affecting populations younger than 12 years. Infants become susceptible to the disease at age 6-12 months after their transplacentally derived immunity wanes. [21] Since the advent of diphtheria vaccination, cases of pediatric disease have declined dramatically. Recently, however, diphtheria has shifted into the adolescent and adult population, most notably those in ages 40 and older accounting for most new cases. [16] This is primarily due to incomplete immunization status, including never being immunized, inefficient vaccine or response to vaccination, and not receiving a booster after previous vaccination. According to immunologic studies, one must have an antitoxin level of greater 0.1 IU/mL for adequate immunity. [22] Additionally, adolescents and adults may exhibit an atypical presentation of the disease, thus potentially obscuring the diagnosis. [9]
Immunization schedules have recently changed requiring a toxoid booster at age 11-12 and every 10 years thereafter. The toxoid booster, without tetanus, is approved for pregnant women if their antitoxin titers are less than 0.1 IU/mL. [22]
-
The characteristic thick membrane of diphtheria infection in the posterior pharynx.
-
Cervical edema and cervical lymphadenopathy from diphtheria infection produce a bull's neck appearance in this child. Source: Public Domain www.immunize.org/images/ca.d/ipcd1861/img0002.htm.
-
Photomicrograph depicts a number of gram-positive Corynebacterium diphtheriae bacteria, which had been stained using the methylene blue technique. The specimen was taken from a Pai's slant culture.







