Practice Essentials
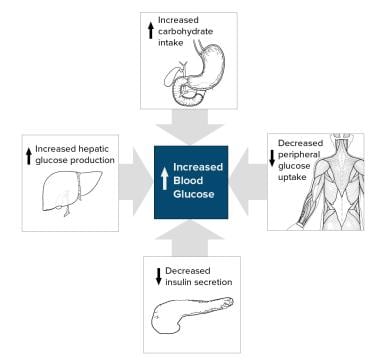
Type 2 diabetes mellitus consists of an array of dysfunctions characterized by hyperglycemia and resulting from the combination of resistance to insulin action, inadequate insulin secretion, and excessive or inappropriate glucagon secretion. See the image below.
Signs and symptoms
Many patients with type 2 diabetes are asymptomatic. Clinical manifestations include the following:
-
Classic symptoms: Polyuria, polydipsia, polyphagia, and weight loss
-
Blurred vision
-
Lower-extremity paresthesias
-
Yeast infections (eg, balanitis in men)
See Presentation for more detail.
Diagnosis
Diagnostic criteria by the American Diabetes Association (ADA) include the following [1] :
-
A fasting plasma glucose (FPG) level of 126 mg/dL (7.0 mmol/L) or higher, or
-
A 2-hour plasma glucose level of 200 mg/dL (11.1 mmol/L) or higher during a 75-g oral glucose tolerance test (OGTT), or
-
A random plasma glucose of 200 mg/dL (11.1 mmol/L) or higher in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis
Whether a hemoglobin A1c (HbA1c) level of 6.5% or higher should be a primary diagnostic criterion or an optional criterion remains a point of controversy.
Indications for diabetes screening in asymptomatic adults includes the following [2, 3, 4, 5] :
-
Sustained blood pressure >135/80 mm Hg
-
Overweight and 1 or more other risk factors for diabetes (eg, first-degree relative with diabetes, BP 140/90 mm Hg or above, and HDL < 35 mg/dL and/or triglyceride level >250 mg/dL)
-
The ADA recommends screening at age 35 years in the absence of the above criteria
See Workup for more detail.
Management
Goals of treatment are as follows:
-
Microvascular (ie, eye and kidney disease) risk reduction through control of glycemia and blood pressure
-
Macrovascular (ie, coronary, cerebrovascular, peripheral vascular) risk reduction through control of lipids and hypertension, smoking cessation
-
Metabolic and neurologic risk reduction through control of glycemia
Recommendations for the treatment of type 2 diabetes mellitus from the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) place the patient's condition, desires, abilities, and tolerances at the center of the decision-making process. [6, 7, 8]
The EASD/ADA position statement contains 7 key points:
Individualized glycemic targets and glucose-lowering therapies
Diet, exercise, and education as the foundation of the treatment program
Use of metformin as the optimal first-line drug unless contraindicated
After metformin, the use of 1 or 2 additional oral or injectable agents, with a goal of minimizing adverse effects if possible
Ultimately, insulin therapy alone or with other agents if needed to maintain blood glucose control
Where possible, all treatment decisions should involve the patient, with a focus on patient preferences, needs, and values
A major focus on comprehensive cardiovascular risk reduction
The 2013 ADA guidelines for self-monitoring of blood glucose (SMBG) frequency focus on an individual's specific situation rather than quantifying the number of tests that should be done. The recommendations include the following [9, 10] :
-
Patients on intensive insulin regimens – Perform SMBG at least before meals and snacks, as well as occasionally after meals; at bedtime; before exercise and before critical tasks (eg, driving); when hypoglycemia is suspected; and after treating hypoglycemia until normoglycemia is achieved.
-
Patients using less frequent insulin injections or noninsulin therapies – Use SMBG results to adjust to food intake, activity, or medications to reach specific treatment goals; clinicians must not only educate these individuals on how to interpret their SMBG data, but they should also reevaluate the ongoing need for and frequency of SMBG at each routine visit.
Approaches to prevention of diabetic complications include the following:
-
HbA1c every 3-6 months
-
Yearly dilated eye examinations
-
Annual microalbumin checks
-
Foot examinations at each visit
-
Blood pressure below 130/80 mm Hg
-
Statin therapy to reduce low-density lipoprotein cholesterol
See Treatment and Medication for more detail.
Background
Type 2 diabetes mellitus consists of an array of dysfunctions characterized by hyperglycemia and resulting from the combination of resistance to insulin action, inadequate insulin secretion, and excessive or inappropriate glucagon secretion. Poorly controlled type 2 diabetes is associated with an array of microvascular, macrovascular, and neuropathic complications.
Microvascular complications of diabetes include retinal, renal, and possibly neuropathic disease. Macrovascular complications include coronary artery and peripheral vascular disease. Diabetic neuropathy affects autonomic and peripheral nerves. (See Pathophysiology and Presentation.)
Unlike patients with type 1 diabetes mellitus, patients with type 2 are not absolutely dependent on insulin for life. This distinction was the basis for the older terms for types 1 and 2, insulin dependent and non–insulin dependent diabetes.
However, many patients with type 2 diabetes are ultimately treated with insulin. Because they retain the ability to secrete some endogenous insulin, they are considered to require insulin but not to depend on insulin. Nevertheless, given the potential for confusion due to classification based on treatment rather than etiology, the older terms have been abandoned. [11] Another older term for type 2 diabetes mellitus was adult-onset diabetes. Currently, because of the epidemic of obesity and inactivity in children, type 2 diabetes mellitus is occurring at younger and younger ages. Although type 2 diabetes mellitus typically affects individuals older than 40 years, it has been diagnosed in children as young as 2 years of age who have a family history of diabetes. In many communities, type 2 diabetes now outnumbers type 1 among children with newly diagnosed diabetes. (See Epidemiology.)
Diabetes mellitus is a chronic disease that requires long-term medical attention to limit the development of its devastating complications and to manage them when they do occur. It is a disproportionately expensive disease; in the United States in 2012, the direct and indirect costs of diagnosed diabetes were estimated to be $245 billion; people with diagnosed diabetes had average medical expenditures 2.3 times those of people without diabetes. [12, 13]
This article focuses on the diagnosis and treatment of type 2 diabetes and its acute and chronic complications, other than those directly associated with hypoglycemia and severe metabolic disturbances, such as hyperosmolar hyperglycemic state (HHS) and diabetic ketoacidosis (DKA). For more information on those topics, see Hyperosmolar Hyperglycemic State and Diabetic Ketoacidosis.
Pathophysiology
Type 2 diabetes is characterized by a combination of peripheral insulin resistance and inadequate insulin secretion by pancreatic beta cells. Insulin resistance, which has been attributed to elevated levels of free fatty acids and proinflammatory cytokines in plasma, leads to decreased glucose transport into muscle cells, elevated hepatic glucose production, and increased breakdown of fat.
A role for excess glucagon cannot be underestimated; indeed, type 2 diabetes is an islet paracrinopathy in which the reciprocal relationship between the glucagon-secreting alpha cell and the insulin-secreting beta cell is lost, leading to hyperglucagonemia and hence the consequent hyperglycemia. [14]
For type 2 diabetes mellitus to occur, both insulin resistance and inadequate insulin secretion must exist. For example, all overweight individuals have insulin resistance, but diabetes develops only in those who cannot increase insulin secretion sufficiently to compensate for their insulin resistance. Their insulin concentrations may be high, yet inappropriately low for the level of glycemia.
A simplified scheme for the pathophysiology of abnormal glucose metabolism in type 2 diabetes mellitus is depicted in the image below.
With prolonged diabetes, atrophy of the pancreas may occur. A study by Philippe et al used computed tomography (CT) scan findings, glucagon stimulation test results, and fecal elastase-1 measurements to confirm reduced pancreatic volume in individuals with a median 15-year history of diabetes mellitus (range, 5-26 years). [15] This may also explain the associated exocrine deficiency seen in prolonged diabetes.
Beta-cell dysfunction
Beta-cell dysfunction is a major factor across the spectrum of prediabetes to diabetes. A study of obese adolescents by Bacha et al confirms what is increasingly being stressed in adults as well: Beta-cell dysfunction develops early in the pathologic process and does not necessarily follow the stage of insulin resistance. [16] Singular focus on insulin resistance as the "be all and end all" is gradually shifting, and hopefully better treatment options that address the beta-cell pathology will emerge for early therapy.
Insulin resistance
In the progression from normal to abnormal glucose tolerance, postprandial blood glucose levels increase first. Eventually, fasting hyperglycemia develops as suppression of hepatic gluconeogenesis fails.
During the induction of insulin resistance (such as occurs with a high-calorie diet, steroid administration, or physical inactivity), increased glucagon levels and increased glucose-dependent insulinotropic polypeptide (GIP) levels accompany glucose intolerance. However, the postprandial glucagonlike peptide-1 (GLP-1) response is unaltered. [17]
Genomic factors
Genome-wide association studies of single-nucleotide polymorphisms (SNPs) have identified a number of genetic variants that are associated with beta-cell function and insulin resistance. Some of these SNPs appear to increase the risk for type 2 diabetes. Over 40 independent loci demonstrating an association with an increased risk for type 2 diabetes have been shown. [18] A subset of the most potent are shared below [19] :
-
Decreased beta-cell responsiveness, leading to impaired insulin processing and decreased insulin secretion (TCF7L2)
-
Lowered early glucose-stimulated insulin release (MTNR1B, FADS1, DGKB, GCK)
-
Altered metabolism of unsaturated fatty acids (FSADS1)
-
Dysregulation of fat metabolism (PPARG)
-
Inhibition of serum glucose release (KCNJ11) [20]
-
Control of the development of pancreatic structures, including beta-islet cells (HHEX) [23]
-
Transport of zinc into the beta-islet cells, which influences the production and secretion of insulin (SLC30A8) [23]
-
Survival and function of beta-islet cells (WFS1) [24]
Susceptibility to type 2 diabetes may also be affected by genetic variants involving incretin hormones, which are released from endocrine cells in the gut and stimulate insulin secretion in response to digestion of food. For example, reduced beta-cell function has been associated with a variant in the gene that codes for the receptor of gastric inhibitory polypeptide (GIPR). [25]
The high mobility group A1 (HMGA1) protein is a key regulator of the insulin receptor gene (INSR). [26] Functional variants of the HMGA1 gene are associated with an increased risk of diabetes.
Amino acid metabolism
Amino acid metabolism may play a key role early in the development of type 2 diabetes. Wang et al reported that the risk of future diabetes was at least 4-fold higher in normoglycemic individuals with high fasting plasma concentrations of 3 amino acids (isoleucine, phenylalanine, and tyrosine). Concentrations of these amino acids were elevated up to 12 years prior to the onset of diabetes. [27] In this study, amino acids, amines, and other polar metabolites were profiled using liquid chromatography tandem mass spectrometry.
Diabetes complications
Although the pathophysiology of the disease differs between the types of diabetes, most of the complications, including microvascular, macrovascular, and neuropathic, are similar regardless of the type of diabetes. Hyperglycemia appears to be the determinant of microvascular and metabolic complications. Macrovascular disease may be less related to glycemia.
Telomere attrition may be a marker associated with presence and the number of diabetic complications. Whether it is a cause or a consequence of diabetes remains to be seen. [28]
Cardiovascular risk
Cardiovascular risk in people with diabetes is related in part to insulin resistance, with the following concomitant lipid abnormalities:
-
Elevated levels of small, dense low-density lipoprotein (LDL) cholesterol particles
-
Low levels of high-density lipoprotein (HDL) cholesterol
-
Elevated levels of triglyceride-rich remnant lipoproteins
Thrombotic abnormalities (ie, elevated type-1 plasminogen activator inhibitor [PAI-1], elevated fibrinogen) and hypertension are also involved. Other conventional atherosclerotic risk factors (eg, family history, smoking, elevated LDL cholesterol) also affect cardiovascular risk.
Insulin resistance is associated with increased lipid accumulation in liver and smooth muscle, but not with increased myocardial lipid accumulation. [29] Persistent lipid abnormalities remain in patients with diabetes despite the use of lipid-modifying drugs, although evidence supports the benefits of these drugs. Statin dose up-titration and the addition of other lipid-modifying agents are needed. [30]
Increased cardiovascular risk appears to begin prior to the development of frank hyperglycemia, presumably because of the effects of insulin resistance. Stern in 1996 [31] and Haffner and D'Agostino in 1999 [32] developed the "ticking clock" hypothesis of complications, asserting that the clock starts ticking for microvascular risk at the onset of hyperglycemia, while the clock starts ticking for macrovascular risk at some antecedent point, presumably with the onset of insulin resistance.
The question of when diabetes becomes a cardiovascular risk equivalent has not yet been settled. Debate has moved beyond automatically considering diabetes a cardiovascular risk equivalent. Perhaps it would be prudent to assume the equivalency with diabetes that is more than 5-10 years in duration.
Cognitive decline
In a cross-sectional study of 350 patients aged 55 years and older with type 2 diabetes and 363 control participants aged 60 years and older without diabetes, diabetic individuals were more likely to have brain atrophy than cerebrovascular lesions, with patterns resembling those of preclinical Alzheimer disease. [33, 34] Type 2 diabetes was associated with hippocampal atrophy; temporal, frontal, and limbic gray-matter atrophy; and, to a lesser extent, frontal and temporal white-matter atrophy.
Type 2 diabetes was also linked with poorer performance on certain cognitive tests. The strength of these associations dropped by almost 50% when adjusted for hippocampal and total gray-matter volumes but was unchanged when adjusted for cerebrovascular lesions or white-matter volume. [33, 34] Patients with type 2 diabetes were more likely to have gray-matter atrophy in several bilateral regions of the cortices, especially in the left hemisphere, similar to the distribution of cortical atrophy described in early Alzheimer disease. [33]
In a 40-month study of 2977 middle-aged and older adults with long-standing type 2 diabetes, depression at baseline was associated with accelerated cognitive decline. [35, 36] The 531 subjects with scores of 10 or higher on the Patient Health Questionnaire Depression Scale at baseline had significantly lower scores on the Digit Symbol Substitution Test (DSST), the Rey Auditory Verbal Learning Test (RAVLT), and the modified Stroop test. Adjustment for other risk factors did not affect the association.
Pulmonary disease
A British study indicated that high blood sugar in type 2 diabetes and prediabetes can directly result in lung complications such as restrictive lung disease, fibrosis, and pneumonia. This was supported by a finding that greater blood glucose levels in type 2 diabetes reduce forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1). Between 16% and 20% of persons with type 2 diabetes have restrictive lung disease, while during the COVID-19 pandemic, lung fibrosis was determined to occur more frequently in individuals with type 2 diabetes than in the general population. [37]
COVID-19
A study reported that out of 178 adult patients hospitalized with coronavirus disease 2019 (COVID-19), at least one underlying condition was found in 89.3%, the most common being hypertension (49.7%), obesity (48.3%), chronic lung disease (34.6%), diabetes mellitus (28.3%), and cardiovascular disease (27.8%). [38]
According to a report by Stokes et al, out of 287,320 US cases of COVID-19 in which the patient’s underlying health status was known, diabetes was the second most common underlying condition (30%), after cardiovascular disease (32%), which in this study included hypertension. [39, 40]
A report by Barrera et al looking at 65 observational studies (15,794 participants) found the overall prevalence of diabetes in patients with COVID-19 to be 12%, with the prevalence being 18% in severe COVID-19. [41, 42]
Results from a study by Guo et al suggested that in patients with COVID-19 infection, the increase in inflammatory and coagulation markers is greater in those with type 2 diabetes mellitus than in individuals without diabetes. This may help to indicate why the risk of more severe disease and death from COVID-19 infection is higher in patients with diabetes. [43, 44]
Secondary diabetes
Various other types of diabetes, previously called secondary diabetes, are caused by other illnesses or medications. Depending on the primary process involved (eg, destruction of pancreatic beta cells or development of peripheral insulin resistance), these types of diabetes behave similarly to type 1 or type 2 diabetes.
The most common causes of secondary diabetes are as follows:
-
Diseases of the pancreas that destroy the pancreatic beta cells (eg, hemochromatosis, pancreatitis, cystic fibrosis, pancreatic cancer)
-
Hormonal syndromes that interfere with insulin secretion (eg, pheochromocytoma)
-
Hormonal syndromes that cause peripheral insulin resistance (eg, acromegaly, Cushing syndrome, pheochromocytoma)
-
Drugs (eg, phenytoin, glucocorticoids, estrogens)
Gestational diabetes
Gestational diabetes mellitus is defined as any degree of glucose intolerance with onset or first recognition during pregnancy (see Diabetes Mellitus and Pregnancy). Gestational diabetes mellitus is a complication of approximately 4% of all pregnancies in the United States. A steady decline in insulin sensitivity as gestation progresses is a normal feature of pregnancy; gestational diabetes mellitus results when maternal insulin secretion cannot increase sufficiently to counteract the decrease in insulin sensitivity.
Subtypes
A study by Ahlqvist et al suggested that type 1 and type 2 diabetes mellitus can actually be divided into five separate types, or clusters, of diabetes. Using six variables to analyze almost 15,000 patients in Sweden and Finland, the investigators came up with the following clusters, the first of which corresponds to type 1 diabetes and the rest of which are subtypes of type 2 diabetes [45, 46] :
-
Severe autoimmune diabetes (SAID) - Essentially corresponding with type 1 diabetes and latent autoimmune diabetes in adults (LADA), this form is characterized by onset at a young age and patients with a relatively low body mass index (BMI), poor metabolic control, and impaired insulin production; in addition, this cluster is positive for glutamic acid decarboxylase antibodies (GADA)
-
Severe insulin-deficient diabetes (SIDD) - This cluster is similar to SAID but is GADA-negative and is characterized by high HbA1c and the greatest risk for diabetic retinopathy among all the clusters
-
Severe insulin-resistant diabetes (SIRD) - This cluster is characterized by insulin resistance and patients with a high BMI and the greatest risk for diabetic nephropathy
-
Mild obesity-related diabetes (MOD) - Patients in this cluster are younger, have obesity, and are not insulin resistant
-
Mild age-related diabetes (MARD) - Patients in this cluster are older, and their metabolic alterations are modest
The investigators maintained that studies in less homogeneous populations are needed to confirm their results but see their report as a “first step towards a more precise, clinically useful stratification” of diabetes. [46]
Etiology
The etiology of type 2 diabetes mellitus appears to involve complex interactions between environmental and genetic factors. Presumably, the disease develops when a diabetogenic lifestyle (ie, excessive caloric intake, inadequate caloric expenditure, obesity) is superimposed on a susceptible genotype.
The body mass index (BMI) at which excess weight increases risk for diabetes varies with different racial groups. For example, compared with persons of European ancestry, persons of Asian ancestry are at increased risk for diabetes at lower levels of overweight. [47] Hypertension and prehypertension are associated with a greater risk of developing diabetes in Whites than in African Americans. [48]
In addition, an in utero environment resulting in low birth weight may predispose some individuals to develop type 2 diabetes mellitus. [49, 50, 51] Infant weight velocity has a small, indirect effect on adult insulin resistance, and this is primarily mediated through its effect on BMI and waist circumference. [52]
Approximately 90% of individuals with type 2 diabetes mellitus are overweight or have obesity. [53] However, a large, population-based, prospective study has shown that an energy-dense diet may be a risk factor for the development of diabetes that is independent of baseline obesity. [54]
A study by Cameron et al indicated that in the United States between 2013 and 2016, obesity was responsible for the development of new-onset diabetes in 41% of adults. The highest attributable rate of obesity-related diabetes was among non-Hispanic White women (53%); non-Hispanic Black men demonstrated the lowest rate, with the attributable fraction being 30%. [55, 56]
Some studies suggest that environmental pollutants may play a role in the development and progression of type 2 diabetes mellitus. [57] A structured and planned platform is needed to fully explore the diabetes-inducing potential of environmental pollutants.
Secondary diabetes may occur in patients taking glucocorticoids or when patients have conditions that antagonize the actions of insulin (eg, Cushing syndrome, acromegaly, pheochromocytoma).
A study by Pauza et al suggested that glucagonlike peptide–1 (GLP-1) is associated with the link between diabetes and hypertension. The investigators found that GLP-1 receptors are expressed on the carotid body and, working with rats, determined that reduced expression of these receptors “is linked to sympathetic hyperactivity in rats with cardiometabolic disease.” Thus, the research indicates that GLP-1 not only plays its known part in glucose control (by stimulating insulin release) but is associated with blood pressure control as well. [58, 59]
Major risk factors
The major risk factors for type 2 diabetes mellitus are the following:
-
Age greater than 45 years (though, as noted above, type 2 diabetes mellitus is occurring with increasing frequency in young individuals)
-
Weight greater than 120% of desirable body weight
-
Family history of type 2 diabetes in a first-degree relative (eg, parent or sibling)
-
Hispanic, Native American, African American, Asian American, or Pacific Islander descent
-
History of previous impaired glucose tolerance (IGT) or impaired fasting glucose (IFG)
-
Hypertension (130/80 mm Hg or above) or dyslipidemia (HDL cholesterol level < 40 mg/dL or triglyceride level >150 mg/dL)
-
History of gestational diabetes mellitus or of delivering a baby with a birth weight of over 9 lb
-
Polycystic ovarian syndrome (which results in insulin resistance)
Genetic influences
The genetics of type 2 diabetes are complex and not completely understood. Evidence supports the involvement of multiple genes in pancreatic beta-cell failure and insulin resistance.
Genome-wide association studies have identified dozens of common genetic variants associated with increased risk for type 2 diabetes. [19] Of the variants thus far discovered, the one with the strongest effect on susceptibility is the transcription factor 7–like 2 (TCF7L2) gene. (For more information, see Type 2 Diabetes and TCF7L2.)
Identified genetic variants account for only about 10% of the heritable component of most type 2 diabetes. [19] An international research consortium found that use of a 40-SNP genetic risk score improves the ability to make an approximate 8-year risk prediction for diabetes beyond that which is achievable when only common clinical diabetes risk factors are used. Moreover, the predictive ability is better in younger persons (in whom early preventive strategies could delay diabetes onset) than in those older than 50 years. [60]
Some forms of diabetes have a clear association with genetic defects. The syndrome historically known as maturity onset diabetes of youth (MODY), which is now understood to be a variety of defects in beta-cell function, accounts for 2-5% of individuals with type 2 diabetes who present at a young age and have mild disease. The trait is autosomal dominant and can be screened for through commercial laboratories.
To date, 11 MODY subtypes have been identified, involving mutations in the following genes [61, 62] :
-
HNF-4-alpha
-
Glucokinase gene
-
HNF-1-alpha
-
IPF-1
-
HNF-1-beta
-
NEUROD1
-
KLF11 [63]
-
CEL [64]
-
PAX4 [65]
-
INS
-
BLK [66]
Most of the MODY subtypes are associated with diabetes only; however, MODY type 5 is known to be associated with renal cysts, [67] and MODY type 8 is associated with exocrine pancreatic dysfunction. [64]
A number of variants in mitochondrial deoxyribonucleic acid (DNA) have been proposed as an etiologic factor for a small percentage of patients with type 2 diabetes. Two specific point mutations and some deletions and duplications in the mitochondrial genome can cause type 2 diabetes and sensorineural hearing loss. [68]
Diabetes can also be a finding in more severe mitochondrial disorders such as Kearns-Sayre syndrome and mitochondrial encephalomyopathy, lactic acidosis, and strokelike episode (MELAS). Mitochondrial forms of diabetes mellitus should be considered when diabetes occurs in conjunction with hearing loss, myopathy, seizure disorder, strokelike episodes, retinitis pigmentosa, external ophthalmoplegia, or cataracts. These findings are of particular significance if there is evidence of maternal inheritance.
A meta-analysis of two studies indicated that a genetically associated low birth weight increases an individual’s risk for developing type 2 diabetes. The report found that for each one-point increase in an individual’s genetic risk score for low birth weight, the type 2 diabetes risk rose by 6%. [69, 70]
Depression
Accumulating evidence suggests that depression is a significant risk factor for developing type 2 diabetes. Pan et al found that the relative risk was 1.17 in women with depressed mood and 1.25 in women using antidepressants. [71] Antidepressant use may be a marker of more severe, chronic, or recurrent depression, or antidepressant use itself may increase diabetes risk, possibly by altering glucose homeostasis or promoting weight gain.
In turn, type 2 diabetes has been identified as a risk factor for the development of depression. Depressive symptoms and major depressive disorder are twice as prevalent in patients with type 2 diabetes as in the general population. [72]
Schizophrenia
Schizophrenia has been linked to the risk for type 2 diabetes. Dysfunctional signaling involving protein kinase B (Akt) is a possible mechanism for schizophrenia; moreover, acquired Akt defects are associated with impaired regulation of blood glucose and diabetes, which is overrepresented in first-episode, medication-naive patients with schizophrenia. [73] In addition, second-generation antipsychotics are associated with greater risk for type-2 diabetes.
Preeclampsia and gestational hypertension
A population-based, retrospective cohort study of 1,010,068 pregnant women examined the association between preeclampsia and gestational hypertension during pregnancy and the risk of developing diabetes post partum. Results showed the incidence rate of diabetes per 1000 person-years was 6.47 for women with preeclampsia and 5.26 for those with gestational hypertension, compared with 2.81 in women with neither condition. Risk was further elevated in women with preeclampsia or gestational hypertension comorbid with gestational diabetes. [74]
COVID-19
Evidence exists that coronavirus disease 2019 (COVID-19) may actually lead to the development of type 1 and type 2 diabetes. One theory is that diabetes arises when severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, binds “to angiotensin-converting enzyme 2 (ACE2) receptors in key metabolic organs and tissues, including pancreatic beta cells and kidneys.” The CoviDiab registry was established by an international group of diabetes researchers to gather data on COVID-19–related diabetes. [75]
A report by Xie and Al-Aly found that among study patients who had survived the first 30 days of COVID-19, the risk for diabetes at 1 year was increased by about 40%. More specifically, the hazard ratios (HRs) for diabetes at 1 year among patients who, during the acute infection, were not hospitalized, were hospitalized, or were admitted to intensive care were 1.25, 2.73, and 3.76, respectively. The investigators stated that diabetes "should be considered as a facet of the multifaceted long COVID syndrome." [76, 77]
A study by Tang et al detected SARS-CoV-2 antigen in pancreatic beta cells, as taken from autopsy samples from individuals who had had COVID-19. The research indicated that insulin expression decreases in SARS-CoV-2–infected beta cells, with these cells possibly undergoing transdifferentiation. [78] A study by Wu et al also indicated that infected beta cells secrete less insulin, with the investigators finding evidence that SARS-CoV-2 can induce beta-cell apoptosis. [79]
A study from the US Centers for Disease Control and Prevention (CDC) indicates that SARS-CoV-2 infection increases the likelihood of diabetes developing in children under age 18 years, more than 30 days post infection. The investigators, using two US health claims databases, reported that pediatric patients with COVID-19 in the HealthVerity database were 31% percent more likely than other youth to receive a new diabetes diagnosis, while those in the IQVIA database were 166% more likely. The study could not specify the type or types of diabetes specifically related to COVID-19, with the report saying that the disease could be causing both type 1 and type 2 diabetes but through differing mechanisms. The researchers suggested, however, that COVID-19 may induce diabetes by directly attacking pancreatic cells that express ACE2 receptors, that it may give rise to diabetes “through stress hyperglycemia resulting from the cytokine storm and alterations in glucose metabolism caused by infection,” or that COVID-19 may cause diabetes via the conversion of prediabetes to diabetes. Whether the diabetes is transient or chronic was also unknown. [80, 81]
However, a study by Cromer et al looked at adult patients with newly diagnosed diabetes mellitus at the time of hospital admission for COVID-19, finding that a number of them subsequently regressed to a state of normoglycemia or prediabetes. The investigators reported that out of 64 survivors in the study with newly diagnosed diabetes (62 of whom had type 2 diabetes), 26 (40.6%) were known to undergo such regression (median 323-day follow-up). [82]
Epidemiology
Occurrence in the United States
According to the CDC's National Diabetes Statistics Report, the crude prevalence of diabetes in the adult US population is 14.7%. It was estimated that 11.3% of the adult population have actually been diagnosed, while 3.4% of adults have undiagnosed diabetes. The prevalence of diabetes rises with age, reaching 29.2% in persons aged 65 years or older. Data employed in the report were drawn from 2017-2020. [83, 84]
Prediabetes, as defined by the American Diabetes Association, is that state in which blood glucose levels are higher than normal but not high enough to be diagnosed as diabetes. It is presumed that most persons with prediabetes will subsequently progress to diabetes. The above-mentioned CDC report found the age-adjusted estimate for the prevalence of prediabetes in the adult US population to be 10.8%. [83, 84]
A study by Andes et al using a cross-sectional analysis of the National Health and Nutrition Examination Survey (2005-2016) indicated that in the United States, prediabetes exists in approximately 1 out of 5 adolescents and 1 out of 4 young adults. [85, 86]
However, a study by Liu et al reported a higher incidence of prediabetes in young people, revealing that in the United States by 2018, approximately 28% of individuals between ages 12 and 19 years had the condition; this was up from less than 12% in 1999. A greater prevalence of prediabetes was found in males in this group and in youth with overweight or obesity. [87, 88]
In 2014, the CDC reported that about 40% of US adults will develop diabetes, primarily type 2, in their lifetime, and that more than 50% of ethnic minorities will be affected. This is substantially higher than previous estimates. The central reason for the increase is obesity. [89, 90]
A study by Ludwig et al found that neighborhoods with high levels of poverty are associated with increases in the incidence of extreme obesity and diabetes. Although the mechanisms behind this association is unclear, further investigation is warranted. [91]
International occurrence
Type 2 diabetes mellitus is less common in non-Western countries where the diet contains fewer calories and daily caloric expenditure is higher. However, as people in these countries adopt Western lifestyles, weight gain and type 2 diabetes mellitus are becoming virtually epidemic.
The 10th edition of the International Diabetes Federation Diabetes Atlas, published in December 2021, reported that worldwide, 1 in 10 adults has diabetes. The data predicted that there would be a global increase in the number of adults with diabetes from 537 million in 2021 to 786 million by 2045, a 46% rise. Although increases are expected throughout the world, Africa, the Middle East, and Southeast Asia are predicted to have the greatest expansion. [92]
Race-related demographics
The prevalence of type 2 diabetes mellitus varies widely among various racial and ethnic groups. The image below shows data for various populations. Type 2 diabetes mellitus is more prevalent among Hispanics, Native Americans, African Americans, and Asians/Pacific Islanders than in non-Hispanic Whites. Indeed, the disease is becoming virtually pandemic in some groups of Native Americans and Hispanic people. The risk of retinopathy and nephropathy appears to be greater in Blacks, Native Americans, and Hispanics.
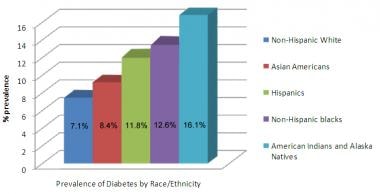 Prevalence of type 2 diabetes mellitus in various racial and ethnic groups in the United States (2007-2009 data).
Prevalence of type 2 diabetes mellitus in various racial and ethnic groups in the United States (2007-2009 data).
In a study by Selvin et al, differences between Blacks and Whites were noted in many glycemic markers and not just the hemoglobin A1c (HbA1c) level. [93] This suggests real differences in glycemia, rather than in the hemoglobin glycation process or erythrocyte turnover, between Blacks and Whites.
Age-related demographics
Type 2 diabetes mellitus occurs most commonly in adults aged 40 years or older, and the prevalence of the disease increases with advancing age. Indeed, the aging of the population is one reason that type 2 diabetes mellitus is becoming increasingly common. Virtually all cases of diabetes mellitus in older individuals are type 2.
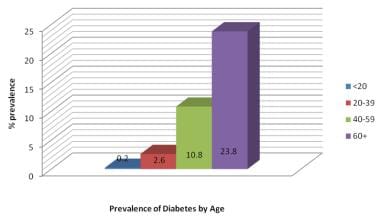
In addition, however, the incidence of type 2 diabetes is increasing more rapidly in adolescents and young adults than in other age groups. The disease is being recognized increasingly in younger persons, particularly in highly susceptible racial and ethnic groups and the obese. In some areas, more type 2 than type 1 diabetes mellitus is being diagnosed in prepubertal children, teenagers, and young adults. The prevalence of diabetes mellitus by age is shown in the image below.
Prognosis
The prognosis in patients with diabetes mellitus is strongly influenced by the degree of control of their disease. Chronic hyperglycemia is associated with an increased risk of microvascular complications, as shown in the Diabetes Control and Complications Trial (DCCT) in individuals with type 1 diabetes [94, 95] and the United Kingdom Prospective Diabetes Study (UKPDS) in people with type 2 diabetes. [96]
Reversion to normal glucose regulation during attempts to prevent progression of pre-diabetes to frank diabetes is a good indicator of slowing disease progression, and it is associated with a better prognosis. [97]
Prognosis in intensive therapy
In the UKPDS, more than 5000 patients with type 2 diabetes were followed up for up to 15 years. Those in the intensely treated group had a significantly lower rate of progression of microvascular complications than did patients receiving standard care. Rates of macrovascular disease were not altered except in the metformin-monotherapy arm in obese individuals, in which the risk of myocardial infarction was significantly decreased.
In the 10-year follow-up to the UKPDS, patients in the previously intensively treated group demonstrated a continued reduction in microvascular and all-cause mortality, as well as in cardiovascular events, despite early loss of differences in glycated hemoglobin levels between the intensive-therapy and conventional-therapy groups. [98] The total follow-up was 20 years, half while in the study and half after the study ended.
Other, shorter studies, such as Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) and the Veterans Affairs Diabetes Trial (VADT), showed no improvement in cardiovascular disease and death with tight control (lower targets than in the UKPDS). [99, 100, 101]
In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, increased mortality was noted among the poorly-controlled patients in the intensive glycemic arm; indeed there was a 66% increase in mortality for each 1% increase in HbA1c; the best outcome occurred among patients who achieved the target of an HbA1c of less than 6%. The excess mortality between the intensive and conventional glycemic arms occurred for A1c above 7%.
Differences between the patient populations in these studies and the UKPDS may account for some of the differences in outcome. The patients in these 3 studies had established diabetes and had a prior cardiovascular disease event or were at high risk for a cardiovascular disease event, whereas patients in the UKPDS study were younger, with new-onset diabetes and low rates of cardiovascular disease.
Early, intensive, multifactorial (blood pressure, cholesterol) management in patients with type 2 diabetes mellitus was associated with a small, nonsignificant reduction in the incidence of cardiovascular disease events and death in a multinational European study. [102] The 3057 patients in this study had diabetes detected by screening and were randomized to receive either standard diabetes care or intensive management of hyperglycemia (target HbA1c < 7.0%), blood pressure, and cholesterol levels.
The benefits of intensive intervention were demonstrated in the Steno-2 study in Denmark, which included 160 patients with type 2 diabetes and persistent microalbuminuria; the mean treatment period was 7.8 years, followed by an observational period for a mean of 5.5 years. Intensive therapy was associated with a lower risk of cardiovascular events, death from cardiovascular causes, progression to end-stage renal disease, and need for retinal photocoagulation. [103]
A British study indicated that the HbA1c level achieved 3 months after the initial diagnosis of type 2 diabetes mellitus predicts subsequent mortality. In other words, according to the report, aggressive lowering of glucose after diagnosis bodes well for long-term survival. (Intensified diabetes control must be introduced gradually in newly diagnosed patients.) [104]
Another study, a review of randomized clinical trials, showed that intensive glycemic control reduces the risk of microvascular complications, but at the expense of increased risk of hypoglycemia. All-cause mortality and cardiovascular mortality in the study did not differ significantly with intensive versus conventional glycemic control; however, trials conducted in usual-care settings showed a reduction in the risk of nonfatal myocardial infarction. [105]
Overall, these studies suggest that tight glycemic control (HbA1c < 7% or lower) is valuable for microvascular and macrovascular disease risk reduction in patients with recent-onset disease, no known cardiovascular diseases, and a longer life expectancy. In patients with known cardiovascular disease, a longer duration of diabetes (15 or more years), and a shorter life expectancy, however, tighter glycemic control is not as beneficial, particularly with regard to cardiovascular disease risk. Episodes of severe hypoglycemia may be particularly harmful in older individuals with poorer glycemic control and existing cardiovascular disease.
A study by Zheng et al indicated that HbA1c levels in persons with diabetes are longitudinally associated with long-term cognitive decline, as found using a mean 4.9 cognitive assessments of diabetes patients over a mean 8.1-year follow-up period. The investigators saw a significant link between each 1 mmol/mol rise in HbA1c and an increased rate of decline in z scores for global cognition, memory, and executive function. Patients in the study had a mean age of 65.6 years. The report cited a need for research into whether optimal glucose control in people with diabetes can affect their cognitive decline rate. [106, 107]
Vascular disease considerations
One prospective study with a long follow-up challenges the concept of coronary disease risk equivalency between nondiabetic patients with a first myocardial infarction and patients with type 2 diabetes but without any cardiovascular disease. The study found that patients with type 2 diabetes had a lower long-term cardiovascular risk compared with patients with a first myocardial infarction. Other studies have similarly questioned this risk equivalency. [108]
Patients with diabetes have a lifelong challenge to achieve and maintain blood glucose levels as close to the reference range as possible. With appropriate glycemic control, the risk of microvascular and neuropathic complications is decreased markedly. In addition, if hypertension and hyperlipidemia are treated aggressively, the risk of macrovascular complications decreases as well.
These benefits are weighed against the risk of hypoglycemia and the short-term costs of providing high-quality preventive care. Studies have shown cost savings due to a reduction in acute diabetes-related complications within 1-3 years after starting effective preventive care. Some studies suggest that broad-based focus on treatment (eg, glycemia, nutrition, exercise, lipids, hypertension, smoking cessation) is much more likely to reduce the burden of excess microvascular and macrovascular events.
Yamasaki et al found that abnormal results on single-photon CT myocardial perfusion imaging in asymptomatic patients with type 2 diabetes indicated a higher risk for cardiovascular events (13%), including cardiac death. Smoking and low glomerular filtration rate were significant contributing factors. [109] However, an earlier study questioned the merit of routine screening with adenosine-stress radionuclide myocardial perfusion imaging (MPI) in otherwise asymptomatic type 2 diabetic patients (the Detection of Ischemia in Asymptomatic Diabetics [DIAD] study). [110]
In both diabetic and nondiabetic patients, coronary vasodilator dysfunction is a strong independent predictor of cardiac mortality. In diabetic patients without coronary artery disease, those with impaired coronary flow reserve have event rates similar to those with prior coronary artery disease, while patients with preserved coronary flow reserve have event rates similar to nondiabetic patients. [111]
Diabetes-associated mortality and morbidity
In 2017, diabetes mellitus was the seventh leading cause of death in the United States. [12] In addition, diabetes is a contributing cause of death in many cases, and it is probably underreported as a cause of death. Overall, the death rate among people with diabetes is about twice that of people of similar age but without diabetes. [112]
Diabetes mellitus causes morbidity and mortality because of its role in the development of cardiovascular, renal, neuropathic, and retinal disease. These complications, particularly cardiovascular disease (approximately 50-75% of medical expenditures), are the major sources of expenses for patients with diabetes mellitus.
Diabetic retinopathy
Diabetes mellitus is the major cause of blindness in adults aged 20-74 years in the United States; diabetic retinopathy accounts for 12,000-24,000 newly blind persons every year. [113] The National Eye Institute estimates that laser surgery and appropriate follow-up care can reduce the risk of blindness from diabetic retinopathy by 90%. [113]
End-stage renal disease
Diabetes mellitus, and particularly type 2 diabetes mellitus, is the leading contributor to end-stage renal disease (ESRD) in the United States. [113] According to the CDC, diabetes accounts for 44% of new cases of ESRD. [112] In 2008, 48,374 people with diabetes in the United States and Puerto Rico began renal replacement therapy, and 202,290 people with diabetes were on dialysis or had received a kidney transplant. [113]
Neuropathy and vasculopathy
Diabetes mellitus is the leading cause of nontraumatic lower limb amputations in the United States, with a 15- to 40-fold increase in risk over that of the nondiabetic population. In 2006, about 65,700 nontraumatic lower limb amputations were performed related to neuropathy and vasculopathy. [113]
Cardiovascular disease
The risk for coronary heart disease (CHD) is 2-4 times greater in patients with diabetes than in individuals without diabetes. Cardiovascular disease is the major source of mortality in patients with type 2 diabetes mellitus. Approximately two thirds of people with diabetes die of heart disease or stroke. Men with diabetes face a 2-fold increased risk for CHD, and women have a 3- to 4-fold increased risk.
Although type 2 diabetes mellitus, both early onset (< 60 y) and late onset (>60 y), is associated with an increased risk of major CHD and mortality, only the early onset type (duration >10 y) appears to be a CHD risk equivalent. [114]
In patients with type 2 diabetes mellitus, a fasting glucose level of more than 100 mg/dL significantly contributes to the risk of cardiovascular disease and death, independent of other known risk factors. [115] This is based on a review of 97 prospective studies involving 820,900 patients.
Data from a large population-based study affirms that worsening glycemic control appears to increase the risk of heart failure. [116]
Adolescents with obesity and obesity-related type 2 diabetes mellitus demonstrate a decrease in diastolic dysfunction. [117] This suggests that they may be at increased risk of progressing to early heart failure compared with adolescents who are either lean or obese but do not have type 2 diabetes mellitus.
Cancer
A 2010 Consensus Report from a panel of experts chosen jointly by the American Diabetes Association and the American Cancer Society suggested that people with type 2 diabetes are at an increased risk for many types of cancer. [118] Patients with diabetes have a higher risk for bladder cancer, particularly those patients who use pioglitazone. [119, 120] Age, male gender, neuropathy, and urinary tract infections were associated with this risk.
In a meta-analysis of 20 publications comprising 13,008 cancer patients with concurrent type 2 diabetes, researchers found that patients treated with metformin had better overall and cancer-specific survival than those treated with other types of glucose-lowering agents. [121, 122] These improvements were observed across cancer subtypes and geographic locations. Risk reduction was significant among patients with prostate, pancreatic, breast, colorectal and other cancers, but not for those with lung cancer. However, it remains unclear whether metformin can modulate clinical outcomes in cancer patients with diabetes.
Pneumonia
A study by López-de-Andrés et al found the incidence of postoperative pneumonia in patients with type 2 diabetes to be 21% higher than in nondiabetic patients, although the risk of inhospital mortality following the development of postoperative pneumonia was no greater in the presence of type 2 diabetes. [123]
COVID-19
A retrospective study by Chen et al of 136 COVID-19 patients with diabetes (primarily type 2 diabetes) found that older age, elevated C-reactive protein, and insulin use were risk factors for mortality. The adjusted odds ratio (OR) for mortality in insulin use was 3.58. It has been questioned, however, whether insulin itself is a risk factor or if the increased mortality reflected the characteristics of the patients taking it. [124, 125]
A study by Bode et al indicated that among patients with COVID-19, the US in-hospital death rate for individuals living with diabetes, patients with an HbA1c of 6.5% or higher, and those with hyperglycemia throughout their stay is 29%, a figure over four times greater than that for patients without diabetes or hyperglycemia. Moreover, the in-hospital death rate for patients with no evidence of preadmission diabetes who develop hyperglycemia while admitted was found to be seven times higher (42%). [126, 127]
A whole-population study from the United Kingdom reported that the risk of in-hospital death for patients with COVID-19 was 2.0 times greater for those with type 2 diabetes and 3.5 times higher for individuals with type 1 diabetes. However, patients under age 40 years with either type of diabetes were at extremely low risk for death. [128, 129]
A retrospective study by Zhu et al found that among individuals with COVID-19, those who also had type 2 diabetes mellitus had a mortality rate of 7.8% (versus 2.7% for those without diabetes), as well as a higher rate of multiple organ injury. However, the investigators also reported that among the patients with type 2 diabetes, the mortality rate was lower in those who, during hospitalization, had well-controlled blood glucose, that is, patients with a glycemic variability within 3.9 to 10.0 mmol/L, than in those with poorly controlled blood glucose, in which the upper limit of glycemic variability extended beyond 10.0 mmol/L. [130, 131]
The aforementioned study by Barrera et al indicated that among COVID-19 patients with diabetes, the unadjusted relative risk for admission to an intensive care unit (ICU) is 1.96, and for mortality, 2.78. [41, 42]
Another study from the United Kingdom found that risk factors for mortality in COVID-19 patients with type 1 or type 2 diabetes include male sex, older age, renal impairment, non-White ethnicity, socioeconomic deprivation, and previous stroke and heart failure. Moreover, patients with type 1 or type 2 diabetes had a significantly greater mortality risk with an HbA1c level of 86 mmol/mol or above, compared with persons with an HbA1c level of 48-53 mmol/mol. In addition, an HbA1c of 59 mmol/mol or higher in patients with type 2 diabetes increased the risk as well. The study also found that in both types of diabetes, BMI had a U-shaped relationship with death, the mortality risk being increased in lower BMI and higher BMI but being reduced between these (25.0-29.9 kg/m2). [132, 129]
A literature review by Schlesinger et al strengthened the association between severe diabetes and COVID-19–related mortality, finding that among study patients with diabetes, the likelihood of death from COVID-19 was 75% greater in chronic insulin users. The study also indicated that the chance of death from COVID-19 is 50% less in individuals undergoing metformin therapy than in other patients with diabetes. The investigators suggested that the medications themselves did not impact survival but were indicators of the severity of diabetes in each group, with the prognosis being poorer among those with more severe diabetes. [133, 134]
A retrospective study by Wang et al indicated that hyperglycemia, even in the absence of diabetes, is an independent predictor of 28-day mortality in patients with COVID-19. The investigators reported that on admission to two hospitals in Wuhan, China, 29.1% of study patients with COVID-19 and no prior diagnosis of diabetes had a fasting blood glucose of at least 7.0 mmol/L. It was believed that the individuals with hyperglycemia included not only persons with undiagnosed diabetes, but also nondiabetic patients with acute stress hyperglycemia. With regard to 28-day mortality, it was determined that the hazard ratio in patients with a fasting blood glucose of 7.0 mmol/L or higher was 2.30. [135, 136]
Similarly, another report found that in study patients with COVID-19 who had a blood glucose level of over 6.1 mmol/L, the risk of disease progression was 58% greater, with the mortality risk being 3.22-fold higher. [137]
A retrospective, multicenter study by Carrasco-Sánchez et al supported these results, indicating that among noncritical patients with COVID-19, the presence of hyperglycemia on hospital admission independently predicts progression to critical status, as well as death, whether or not the patient has diabetes. The in-hospital mortality rate in persons with a blood glucose level of higher than 180 mg/dL was 41.1%, compared with 15.7% for those with a level below 140 mg/dL. Moreover, the need for ventilation and intensive care unit admission were also greater in the presence of hyperglycemia. The report involved over 11,000 patients with confirmed COVID-19, only about 19% of whom had diabetes. [138, 139]
In contrast to the above research, a report by Klonoff et al on over 1500 US patients with COVID-19 found no association between hyperglycemia on hospital admission and mortality, in non-ICU patients. However, the in-hospital mortality rate was significantly greater in such patients if they had a blood glucose level above 13.88 mmol/L on the second or third hospital day, compared with those with a level below 7.77 mmol/L. Findings for patients admitted directly to the ICU differed from these, with the investigators determining that mortality was associated with the presence of hyperglycemia on admission but was not significantly linked with a high glucose level on the second hospital day. [140, 141]
A study by Sardu et al indicated that in hospitalized patients with COVID-19 and moderately severe pneumonia, those with diabetes and those who are hyperglycemic are at higher risk of severe disease than are normoglycemic patients without diabetes. Moreover, among the patients in the study with hyperglycemia, the risk of severe disease was lower in those who were treated with insulin infusion, providing further evidence of the importance of in-hospital glucose control. [125, 142]
A study by Cariou et al reported that in patients with diabetes hospitalized for COVID-19, a positive, independent association was found between higher body mass index (BMI) and risk of tracheal intubation and/or death within 7 days. The median BMI in patients who suffered this outcome was 29.1 kg/m2, compared with 28.1 kg/m2 in those who did not. However, an association was not found between long-term glucose control and 7-day tracheal intubation and/or death. Regarding specific outcome rates, the study, in which 88.5% of the diabetes cases were type 2 diabetes, reported that 20.3% of the patients with diabetes who were hospitalized with COVID-19 underwent tracheal intubation within 7 days, while 10.6% died within this time. [143, 144]
A French study, by Wargny et al, indicated that among patients with diabetes who are hospitalized with COVID-19, approximately 20% will die within 28 days. Individuals particularly at risk for mortality over this 4-week period include patients of advanced age, as well as those with a history of microvascular complications (especially those who have had kidney or eye damage), who have dyspnea on admission or inflammatory markers (increased white blood cell [WBC] count, raised C-reactive protein, elevated aspartate transaminase), or who have undergone routine insulin and statin treatment. It should be kept in mind, however, that the data was gathered between March 10 and April 10, 2020, with a statement from Diabetes UK explaining that in people with diabetes, COVID-19–associated mortality has decreased over time as treatment has improved. [145, 146]
The Centers for Disease Control and Prevention (CDC) includes type 2 diabetes in the list of conditions that increase the likelihood of severe illness in persons with COVID-19, and type 1 diabetes in the list of conditions that may increase this likelihood. [147]
Pregnancy outcome
Untreated gestational diabetes mellitus can lead to fetal macrosomia, hypoglycemia, hypocalcemia, and hyperbilirubinemia. In addition, mothers with gestational diabetes mellitus have increased rates of cesarean delivery and chronic hypertension.
Despite advanced age, multiparity, obesity, and social disadvantage, patients with type 2 diabetes were found to have better glycemic control, fewer large-for-gestational-age infants, fewer preterm deliveries, and fewer neonatal care admissions compared with patients with type 1 diabetes. This suggests that better tools are needed to improve glycemic control in patients with type 1 diabetes. [148] (For more information, see Diabetes Mellitus and Pregnancy.)
Patient Education
No longer is it satisfactory to provide patients who have diabetes with brief instructions and a few pamphlets and expect them to manage their disease adequately. Instead, education of these patients should be an active and concerted effort involving the physician, nutritionist, diabetes educator, and other health professionals. Moreover, diabetes education needs to be a lifetime exercise; believing that it can be accomplished in 1 or 2 encounters is misguided.
A randomized, controlled trial found that for patients with poorly controlled diabetes, individual attention and education is superior to group education. [149] Similarly, a diabetes education and self-management group program in the UK for newly diagnosed patients failed to yield significant benefits. [150] Nonphysician health professionals are usually much more proficient at diabetes education and have much more time for this very important activity.
A systematic review suggested that patients with type 2 diabetes who have a baseline HbA1c of greater than 8% may achieve better glycemic control when given individual education rather than usual care. Outside that subgroup, however, the report found no significant difference between usual care and individual education. In addition, comparison of individual education with group education showed equal impact on HbA1c at 12-18 months. [151]
Patient education is an immensely complex topic, however. The clinical impression of most experts in the field is that there is merit in the provision of careful diabetes education at all stages of the disease.
-
Simplified scheme for the pathophysiology of type 2 diabetes mellitus.
-
Prevalence of type 2 diabetes mellitus in various racial and ethnic groups in the United States (2007-2009 data).
-
Prevalence of diabetes mellitus type 2 by age in the United States (2007 estimates).
-
Possible physical examination findings in patients with type 2 diabetes mellitus.
-
Diagnostic criteria (American Diabetes Association) for diabetes mellitus type 2.
-
Major findings from the primary glucose study in the United Kingdom Prospective Diabetes Study (UKPDS).
-
Results from metformin substudy in the United Kingdom Prospective Diabetes Study (UKPDS).
-
Findings from the blood pressure substudy in the United Kingdom Prospective Diabetes Study (UKPDS).
-
Laboratory monitoring guidelines for patients with type 2 diabetes mellitus.
-
American Diabetes Association guidelines for low-density lipoprotein cholesterol in diabetes mellitus type 2.
-
Treatment of type 2 diabetes mellitus.
-
Types of insulin. Premixed insulins can be assumed to have a combination of the onset, peak, and duration of the individual components.
-
Simplified scheme for using insulin in treating patients with type 2 diabetes mellitus.
-
Simplified scheme of idealized blood glucose values and multiple dose insulin therapy in type 2 diabetes mellitus.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Pharmacologic Therapy
- Management of Glycemia
- Dietary Modifications
- Activity Modifications
- Bariatric Surgery
- Laboratory Monitoring
- Monitoring for Diabetic Complications
- Management of Hypertension
- Management of Dyslipidemia
- Management of Coronary Heart Disease
- Management of Ophthalmologic Complications
- Management of Diabetic Neuropathy
- Management of Infections
- Management of Intercurrent Medical Illness
- Management of Critical Illness
- Pharmacologic Considerations in Surgery
- Prevention of Type 2 Diabetes Mellitus
- Stroke Prevention in Diabetes
- Consultations
- Show All
- Guidelines
- Medication
- Medication Summary
- Antidiabetics, Biguanides
- Antidiabetics, Sulfonylureas
- Antidiabetics, Meglitinide Derivatives
- Antidiabetics, Alpha-Glucosidase Inhibitors
- Antidiabetics, Thiazolidinediones
- Antidiabetics, Glucagonlike Peptide-1 Agonists
- Dual GIP/GLP-1 Agonists
- Antidiabetics, Dipeptidyl Peptidase IV Inhibitors
- Antidiabetics, Amylinomimetics
- Selective Sodium-Glucose Transporter-2 Inhibitors
- Bile Acid Sequestrants
- Antidiabetics, Rapid-Acting Insulins
- Antidiabetics, Short-Acting Insulins
- Antidiabetics, Intermediate-Acting Insulins
- Antidiabetics, Long-Acting Insulins
- Dopamine Agonists
- Show All
- Questions & Answers
- Media Gallery
- References