Practice Essentials
Procedural sedation may be defined as the administration of sedative or dissociative agents, with or without analgesics, to induce a state that allows the patient to tolerate unpleasant procedures while maintaining cardiorespiratory function. Specifically, the drugs, doses, and techniques used are not likely to produce a loss of protective airway reflexes.
Indications for emergency department sedation
The function of sedation is management of anxiety, pain, and control of excessive motion. Diagnostic procedures for which emergency department (ED) sedation may be indicated include the following:
-
Lumbar puncture
-
Arthrocentesis
-
Bone marrow biopsy
-
Sexual assault examination
-
Radiologic evaluation (CT, MRI)
Therapeutic procedures for which ED sedation may be indicated include the following:
-
Suturing
-
Wound care
-
Abscess incision and drainage
-
Fracture reduction
-
Dislocation
-
Foreign body removal
-
Burn debridement
-
Tube thoracostomy
-
Any other painful procedure
-
Multiple procedures (eg, IV line placement and urethral catheterization) [1]
Safety considerations
Discussion about the risks, benefits, and alternatives with the parent or guardian is necessary before initiation of procedural sedation. The need for written consent may be determined by an institutional, local, or state mandate.
Children of all ages should be NPO for clear liquids 2 hours before undergoing sedation. Recommendations for duration of NPO for solid food and nonclear liquids (eg, infant formula, milk) vary by age, as follows:
-
< 6 months: 4-6 hours
-
6-36 months: 6 hours
-
>36 months: 6-8 hours
Suction and airway equipment should be at the bedside and ready to use if necessary. Equipment should include the following:
-
An appropriately sized positive-pressure oxygen delivery system
-
Suction apparatus
-
Suction catheters (eg, tonsil, Yankauer)
The following equipment should be readily available:
-
Age-appropriate equipment for measuring blood pressure and oxygen saturation
-
A crash cart with age-appropriate drugs and equipment
Preprocedural evaluation
Important elements of the history are as follows:
-
Past medical history, especially with respect to previous sedation or anesthesia
-
Last solid and liquid oral intake
-
Recent illness
-
Medication or drug use
-
Allergies or adverse reaction
-
Pertinent family history
-
Pregnancy status (for postmenarchal females)
-
Upper respiratory infection (URI) symptoms; history of reactive airway disease
Elements of the physical examination are as follows:
-
Age
-
Weight
-
Vital signs
-
Airway examination (head, ears, eyes, nose, and throat [HEENT] and pulmonary examination)
-
Cardiovascular examination
-
Neurologic/mental status
-
Size and location of injury and neurologic status distal to it
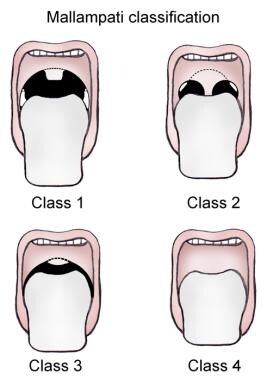
The physical examination focuses on the airway, especially for anatomic variations. The use of the Mallampati classification for assessment helps identify patients in whom airway management is likely to prove difficult (see the image below).
Intraprocedural monitoring
Monitoring during procedural sedation usually should include the following:
-
Maintain continuous oxygen saturation and heart rate monitoring
-
Record vital signs and blood pressure every 15 minutes for conscious sedation and every 5 minutes for deep sedation
-
Record drug dose and time administered
-
Record state of consciousness and response to stimulation
-
Consider end-tidal CO2 monitoring
-
Consider capnography and bispectral index monitoring for patients in the operating room
Sedative medications and reversal agents
Medications for procedural sedation include the following:
-
Opioid analgesics – Morphine sulfate, fentanyl
-
Benzodiazepines – Midazolam, diazepam
-
Barbiturates – Pentobarbital, methohexital, thiopental
-
Miscellaneous agents – Nitrous oxide, ketamine, propofol, dexmedetomidine
Although the medication dose is calculated based on weight, the response can vary significantly from one child to the next. Appropriate actions include the following:
-
Consider starting with the lowest recommended dose (or even half that) and titrate as needed
-
Keep reversal agents, when available, at the bedside, and double-check their proper doses
Reversal agents include the following:
-
Opioids – Naloxone
-
Benzodiazepines - Flumazenil
Postprocedural care
After the completion of the procedure, keep recording vital signs until the patient responds appropriately to a voice or gentle stimulation. Sedation is stimulus-dependent; accordingly, when the procedure is completed, the child is likely to become more sedated than during the procedure, which can lead to hypoventilation and hypoxia.
In general, discharge criteria should include the following:
-
The child’s vital signs should be within 15% of admission readings (either above or below)
-
The child should be ambulatory as appropriate for his or her age, without assistance
-
The child should be able to ingest and retain oral fluids
Some agents are associated with specific aftercare needs. For example, ketamine may cause ataxia for 12-24 hours, and the child’s activities should be restricted during this period to prevent further injury.
Guidelines
The American Academy of Pediatrics (AAP) and the American Academy of Pediatric Dentistry (AAPD) have issued updated clinical guidelines on the monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures.
The guidelines include the following [2, 3] :
-
Practitioners who administer moderate sedation need to have the skills to rescue a child with apnea, laryngospasm and airway obstruction, and to perform successful bag/mask ventilation.
-
Those administering deep sedation also must be able to perform tracheal intubation and cardiopulmonary resuscitation.
-
Additionally, the skilled observer for either moderate or deep sedation must be trained in Pediatric Advanced Life Support.
-
The use of capnography is highly encouraged for children who are moderately sedated and required for those who are deeply sedated.
Overview
Pain and anxiety are common problems in the emergency department (ED). By relieving pain, the emergency provider can render the patient less anxious and more cooperative, thereby potentially achieving a better outcome. Fear, anxiety, and early developmental stage may make it difficult for a child to cooperate with necessary procedures. Failure to cooperate is likely to result in a suboptimal outcome.
Pain in children historically has been underreported, undertreated, and misunderstood. Until comparatively recently, children too young to verbalize were also considered too young to experience pain or fear, and they often received no analgesia, even after major surgery. However, it is now known that even neonates show a physiologic response to painful stimuli. In addition, research has shown that children often do not receive the same treatment as adults with similar painful conditions. Age is apparently a risk factor for oligoanalgesia.
Unfortunately, the current practice of many EDs is not standardized. Many centers and practitioners do not use premedication for children undergoing painful procedures. Reasons for inadequate treatment include failure to recognize pain, ignorance about drugs and dosages, fear of adverse cardiovascular effects, and fear of delay in treatment and disposition.
The practice of pediatric sedation is evolving. [4, 5] Accordingly, this article’s objectives are as follows:
-
To discuss current indications for and goals of sedation in children
-
To emphasize proper preparation and monitoring during procedures
-
To discuss methods of drug administration and specific agents
Sedation Terminology
Although analgesia, anesthesia, and sedation are sometimes conflated, the 3 terms are not synonymous. Anesthesia may include any of the following components:
-
Analgesia
-
Sedation
-
Amnesia
-
Muscle relaxation
Sedation is a continuum. Conscious sedation (in which the patient remains awake) may lead to deep sedation. Deep sedation, in turn, may lead to general anesthesia, which may lead to cardiorespiratory compromise and loss of airway protective reflexes.
The terms used to describe sedation vary. What is more, the health care providers who practice pediatric sedation come from several different fields, including emergency medicine, anesthesiology, intensive care medicine, and radiology. The American College of Emergency Physicians (ACEP), the American Academy of Pediatrics (AAP), and the American Society of Anesthesiology (ASA) have all established guidelines. Consequently, a potential for different practice models exists.
In April 2009, the Consensus Panel on Sedation Research of Pediatric Emergency Research Canada (PERC) and the Pediatric Emergency Care Applied Research Network (PECARN) published recommendations for standardizing terminology and reporting adverse events that involve procedural sedation and analgesia in children. [6] These recommendations will help guide monitoring and quality assurance of pediatric procedural sedation in emergency departments (EDs).
The new recommendation was based upon a systematic review of the literature. The standardization of terminology and the definition of adverse reactions were reached by consensus. An adverse reaction was defined as an event that required intervention from the physician. An adverse reaction had occurred when the physician acted upon specific event(s), such as respiratory compromise, vomiting, cardiovascular compromise, excitatory movements, adverse behavioral reactions, or permanent complication. [6]
For the purposes of the ED practitioner, the term procedural sedation is the most appropriate one to use. Previously used terms, such as conscious sedation and moderate sedation, are misnomers.
Procedural sedation may be defined as the administration of sedative or dissociative agents, with or without analgesics, to induce a state that allows the patient to tolerate unpleasant procedures while maintaining cardiorespiratory function. Procedural sedation and analgesia is intended to result in a depressed level of consciousness that allows the patient to maintain airway control independently and continuously. Specifically, the drugs, doses, and techniques used are not likely to produce a loss of protective airway reflexes.
Factors Affecting Pain Response
The following 4 myths regarding children and pain management are still commonly encountered:
-
Children’s immature central nervous system (CNS) cannot experience pain
-
Children have no memory of pain
-
A given injury elicits a "correct" amount of pain
-
Children easily become addicted to opioids
The following 4 truths are still insufficiently appreciated:
-
Even neonates demonstrate behavioral and hormonal changes in response to painful procedures
-
Children do not have to understand the meaning of pain to experience pain
-
Preemptive analgesia/anesthesia may decrease postinjury opioid requirements
-
A child is likely to require deep sedation in many situations where an adult would require minimal or no sedation
Various individual internal and external factors determine how a child responds to painful procedures and thus affect the decision whether to premedicate the child. Individualized dosing and titratable agents are often necessary.
Internal factors include the child’s age, developmental level, and previous experience. Some children have previously had unpleasant experiences at the hospital and are difficult to manage long before any noxious stimuli occur. These children typically require sedation even when other patients who are the same age may not.
External factors have as much, if not more, influence on a child’s behavior. These include parental interactions with the child, preparation for the procedure, the clinician’s skill, and the physical setting where the procedure is performed. Spending a few minutes preparing the anxious child for a procedure is always in the practitioner’s best interest. Attempting to build trust and being honest about what will and will not hurt may go a long way toward ensuring cooperation.
Indications for Emergency Department Sedation
The function of sedation is management of anxiety, pain, and control of excessive motion. Diagnostic procedures for which emergency department (ED) sedation may be indicated include the following:
-
Arthrocentesis
-
Bone marrow biopsy
-
Sexual assault examination
-
Radiologic evaluation (computed tomography [CT], magnetic resonance imaging [MRI])
Therapeutic procedures for which ED sedation may be indicated include the following:
-
Suturing
-
Wound care
-
Abscess incision and drainage
-
Fracture reduction
-
Dislocation
-
Foreign body removal
-
Burn debridement
-
Tube thoracostomy
-
Any other painful procedure
Procedural sedation is commonly used in major procedures (eg, fracture reduction), but it should also be considered when multiple procedures (eg, intravenous [IV] line placement, lumbar puncture, and urethral catheterization) are being performed on a child. Procedural sedation avoids the need for prolonged restraint and lessens the stress of the procedure on the patient and family members. [1]
Use of anesthetic agents in the ED continues to be an issue at many institutions. To minimize controversy, it is advantageous to anticipate and manage adverse reactions. Many problems can be avoided if individuals with advanced airway skills are immediately available, skilled personnel are present to monitor the patient, appropriate monitoring devices are used, and written policies and procedures are in place.
Many hospitals have a credentialing process that helps to ensure knowledge of the procedure. In many institutions, the ED pharmacopeia is restricted, and specific agents (eg, ketamine, propofol) are unavailable or are approved for use only by anesthesiologists.
Preparing for Sedation
The length of the procedure, the duration of the medication’s effect, and the potential for side effects dictate decisions regarding the type of medication employed and the route by which it is administered. In addition, the setting in which the medications are used play a role in the decision-making process. Customizing the choices is important.
Dosing considerations
Analgesia and sedation should be appropriate for the degree of insult. Although the medication dose is calculated based on weight, the response can vary significantly from one child to the next. Flexibility and careful titration are crucial.
Most analgesics and sedatives in children have a range of acceptable doses, and the emergency department (ED) practitioner may prefer to start with the lowest recommended dose (or even half that) and titrate as needed. Reversal agents, when available, should be kept at the bedside, and their proper doses should be double checked.
Drug absorption, distribution, and elimination can vary with age. Water makes up 70% of body weight in neonates, compared with 55% in adults. Lipid versus water solubility affects the distribution of the agents used. In addition, renal and hepatic elimination improve with age.
An additional method of analgesia can be achieved via intranasal administration of opioids, offering pain relief without the pain associated with intramuscular (IM) administration or intravenous (IV) placement. Saunders et al were able to achieve adequate analgesia with a single intranasal dose of fentanyl in children undergoing orthopedic reduction. [7]
Safety considerations
Safety during pediatric sedation has been an increasing topic of interest in the ED. The most common reasons for adverse events are operator error, lack of familiarity with agents being used, lack of rescue systems, and delay in airway and ventilatory support.
With proper preparation and planning, the incidence of adverse events is low. At one institution, the incidence of adverse events trended downward with the implementation of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) guideline in 2001. Whereas most such studies have been performed in academic centers, one study reported similar findings in community-based EDs.
Guidelines established by the American College of Emergency Physicians (ACEP), the American Academy of Pediatrics (AAP), and the American Society of Anesthesiologists (ASA) evaluate the risk of procedural sedation by assessing key elements of the history and physical examination. Adherence to these guidelines has lowered the risk of complications. Another component is division of responsibilities of the personnel. Distinct personnel need to be responsible for sedation, monitoring, and performing the procedure.
Discussion about the risks, benefits, and alternatives with the parent or guardian is necessary before initiation of procedural sedation. However, written consent is not absolutely necessary. JCAHO has not set forth a requirement. The need for written consent may be determined by an institutional, local, or state mandate.
Recommendations for duration of nothing-by-mouth status before elective procedures have been published (see Table 1 below). The purpose of such recommendations is to prevent aspiration secondary to delayed gastric emptying.
Table 1. AAP/ASA Recommendations for Duration of NPO Before Elective Procedures (Open Table in a new window)
Age |
Solid and Nonclear Liquids* |
Clear Liquids |
< 6 mo |
4-6 h† |
2 h |
6-36 mo |
6 h |
2 h |
> 36 mo |
6-8 h‡ |
2 h |
*Infant formula, breast milk, nonhuman milk. †4 h, according to AAP guidelines. ‡8 h, according to AAP guidelines. AAP = American Academy of Pediatrics; ASA = American Society of Anesthesiologists; NPO = nil per os (nothing by mouth). Adapted from Ann Emerg Med. 2003;42:636-646. |
||
The above recommendations are based on expert opinions. Pooled general anesthesia data indicate that the risk of aspiration is low. Aspirations occur primarily during intubation and extubation, both of which are unlikely events during ED procedural sedation. To date, there have been no reported cases of aspiration arising from the ED. Thus, the best course of action is based on the risk and benefits of the procedure and sedation for the patient.
The final decision to proceed is determined by the necessity of the procedure. Just as important, the decision to delay or abort procedural sedation is dictated by the risk to the patient. Although a number of investigators have tried to address NPO status for ED patients undergoing procedural sedation, no conclusions can be drawn at present, except that adverse events are extremely low regardless of NPO status and that risk is related more to the depth of sedation. A study by Beach et al suggested that NPO status for liquids and solids is not an independent predictor of major complications or aspiration in this sedation/anesthesia data set. [8] A secondary analysis of a multicenter prospective cohort study that included 6,183 children who received procedural sedation also found no association between fasting duration and any type of adverse event. [9]
Even so, it is prudent to expect the worst and to have suction and airway equipment at the bedside and ready to use if necessary. Equipment should include an appropriately sized positive-pressure oxygen delivery system, suction apparatus, and suction catheters (eg, tonsil, Yankauer). Age-appropriate equipment for measuring blood pressure and oxygen saturation should be available, and continuous oxygen saturation monitoring is recommended. A crash cart with age-appropriate drugs and equipment should be readily available.
Note that the above recommendations refer only to medications administered with the goal of sedating a child. Medication used for the sole purpose of analgesia (eg, a narcotic alone) should not require the continuous presence of nursing staff or continuous oxygen saturation monitoring.
Other considerations for adverse events are incorrect dosage calculations and failure to recognize potential drug interactions. One of these reactions is between fentanyl and midazolam. An increase in the respiratory depressant effect occurs. The use of 3 or more medications significantly increases the possibility of an adverse reaction.
A study by Havidich et al found that patients born preterm are nearly twice as likely to develop sedation/anesthesia adverse events, and this risk continues up to 23 years of age. Airway and respiratory adverse events were most commonly reported. [10, 11]
Preprocedural Evaluation
An evaluation of a patient for procedural sedation begins with a thorough history and a careful physical examination.
Important elements of the history are as follows:
-
Past medical history, especially with respect to previous sedation or anesthesia
-
Last solid and liquid oral intake
-
Recent illness
-
Medication or drug use
-
Allergies or adverse reaction
-
Pertinent family history
-
Pregnancy status (for postmenarchal females)
-
Upper respiratory infection (URI) symptoms; history of reactive airway disease
Elements of the physical examination are as follows:
-
Age
-
Weight
-
Vital signs
-
Airway examination (head, ears, eyes, nose, and throat [HEENT] and pulmonary examination)
-
Cardiovascular examination
-
Neurologic/mental status
-
Size and location of injury and neurologic status distal to it
The physical examination focuses on the airway, especially for anatomic variations. The use of the Mallampati classification (see the image below) for assessment helps identify patients in whom airway management is likely to prove difficult.
The ASA physical status classification was developed for patients undergoing general endotracheal anesthesia. It includes the following classes:
-
I - Normal healthy patient
-
II - Patient with mild systemic disease
-
III - Patient with severe systemic disease
-
IV - Patient with severe systemic disease that is a constant threat to life
-
V - Moribund patient not expected to survive without operation
-
E - Emergent procedure
This classification stratifies the risk of complications from a procedural sedation. Class I and II patients are the best candidates for general endotracheal anesthesia. Whether this classification can be accurately extrapolated for procedural sedation in the ED is unknown.
The existence of a preexisting physical or psychological condition is not an absolute contraindication for procedural sedation. Rather, it should help guide the clinician in carefully selecting the most appropriate medication(s) and route of administration.
Laboratory workup has no role before procedural sedation.
A study that evaluated the impact of obesity on adverse events and required interventions during pediatric procedural sedation found that obesity is an independent risk factor for adverse respiratory events during procedural sedation and is associated with an increased frequency of airway interventions. Extra care and attentiveness should be applied when sedating these patients. [12]
A study set to determine whether critically ill children managed with a nurse-implemented, goal-directed sedation protocol, experience fewer days of mechanical ventilation than patients receiving usual care. The authors concluded that among children undergoing mechanical ventilation for acute respiratory failure, the use of a sedation protocol compared with usual care did not reduce the duration of mechanical ventilation however exploratory analyses of secondary outcomes suggest a complex relationship among wakefulness, pain, and agitation. [13, 14]
Intraprocedural Monitoring
Monitoring during procedural sedation usually should include the following:
-
Maintain continuous oxygen saturation and heart rate monitoring
-
Record vital signs and blood pressure every 15 minutes for conscious sedation and every 5 minutes for deep sedation
-
Record drug dose and time administered
-
Record state of consciousness and response to stimulation
-
Consider end-tidal CO2 monitoring
-
Consider capnography and bispectral index monitoring as noninvasive alternative or adjunctive methods of monitoring patients in the operating room (OR); capnography measures end-tidal CO2 concentration during ventilation, identifies hypoventilation and apnea at an earlier stage; bispectral index monitoring measures the depth of sedation from electroencephalographic (EEG) signals
Sedative Medications
Opioid analgesics
Opioid analgesic agents are frequently used to control pain. They may be given either alone or in combination with sedative-anxiolytic agents. In some scenarios, they can be employed as hypnotic agents; however, the risk of respiratory depression is greater in this setting. The analgesic effect occurs at the mu opioid receptor.
Other opioid receptors (eg, kappa, delta) have been implicated in other effects (ie, some sedation and no amnestic properties). These effects are dose-related.
Whenever opioid analgesics are used, a reversal agent should be readily available. Naloxone is an opioid reversal agent that can be administered as 0.1 mg/kg intravenously (IV), intramuscularly (IM), subcutaneously (SC), or endotracheally (ET) every 2-3 minutes until response in children aged 5 years or younger or weighing 20 kg or less. In children older than 5 years or weighing more than 20 kg, the dosage is 2 mg IV, IM, SC, or ET every 2-3 minutes until response. Naloxone’s half-life is 1-2 hours. Rebound sedation and apnea may occur.
Morphine sulfate
Morphine sulfate is indicated for analgesia because of its reliable and predictable effects, good safety profile, and ease of reversibility with naloxone. It elicits analgesia and possesses some sedative effect but has no amnestic properties. Its peak effect is observed 15-30 min after IV administration and 30-60 min after IM administration.
For procedural analgesia and sedation in children, morphine sulfate is given in a dosage of 0.08-0.1 mg/kg/dose IV, IM, or SC before the procedure and every 5-10 minutes as needed.
Fentanyl
Fentanyl is a synthetic opioid that is 75-200 times more potent than morphine sulfate and has a much shorter half-life. It has less hypotensive effects than morphine does and is safer in patients with hyperactive airway disease because there is minimal to no associated histamine release. By itself, fentanyl causes little cardiovascular compromise, although addition of benzodiazepines or other sedatives may result in decreased cardiac output and blood pressure.
Because of its short duration of action (30-60 minutes) and easy titratability, fentanyl is an excellent choice for pain management and sedation. Its onset of action is immediate after IV administration. It is easily and quickly reversed by naloxone.
In children younger than 6 years, fentanyl is given as 0.3-1.5 µg/kg/dose by slow IV push (over 1-2 minutes); this may be repeated every 1-2 hours. In children aged 6 years and older, it is given as 1-5 µg/kg/dose by slow IV push (over 1-2 minutes); the IV dose may be repeated every 1-2 hours (the dose may range from 1 to 10 µg/kg/dose).
Benzodiazepines
Benzodiazepines are used as sedative-hypnotic agents. They have anxiolytic, amnestic, and skeletal muscle relaxant properties. However, they do not have any analgesic properties. They exert effects on gamma-aminobutyric acid (GABA) receptors and potentiate GABA neuron inhibitory actions, as well as result in chlorine channel opening and postsynaptic neuronal hyperpolarization.
Midazolam is commonly used because of its short half-life and prompt onset of action. Diazepam is also used, but it has a long half-life and active metabolites. Lorazepam is a poor choice for procedural sedation, because of its long duration of action.
The benzodiazepine reversal agent is flumazenil; the pediatric dose is 0.01-0.02 mg/kg IV, which may be repeated every minute to a maximum cumulative dose of 1 mg. Flumazenil can precipitate seizures in patients who have ingested tricyclic antidepressants (TCAs) or are long-term benzodiazepine users.
Midazolam
Midazolam is a favored sedative-hypnotic agent for procedural sedation because its water solubility allows it to be administered via several different routes (eg, oral, IV, IM, intranasal, and rectal).
Midazolam has a rapid onset of action when administered IV (2-5 min), is easily titrated, is associated with less pain at the injection site, and has a shorter duration of action than other commonly used benzodiazepines. The dose-response curve is highly variable in children; weight-based dosing produces variable levels of sedation in agitated children of the same weight; this is common with IM and PO dosing.
Dosing in children younger than 6 months has not been established. When midazolam is given IV, 2-3 minutes should be allowed to elapse after a dose before additional doses are administered.
In children aged 6 months to 12 years, midazolam is given as 0.05-0.1 mg/kg IV, titrated to the desired effect. Younger children (ie, < 5 years) may require larger cumulative doses, as high as 0.6 mg/kg or 6 mg.
In children older than 12 years, midazolam is given as 0.01-0.05 mg/kg IV (approximately 0.5-4 mg); the dose may be given slowly or infused over several minutes and may be repeated every 10-15 minutes. Do not exceed a cumulative dose of 10 mg. The oral dose is 0.25-1 mg/kg/dose, not to exceed 20 mg/dose. The IM dose is 0.1-0.15 mg/kg/dose 30-60 minutes before the procedure or operation, not to exceed 10 mg/dose. The intranasal dose is 0.2-0.5 mg/kg.
Diazepam
Diazepam modulates the postsynaptic effects of GABA-A transmission, resulting in an increase in presynaptic inhibition. It appears to act on part of the limbic system, the thalamus, and the hypothalamus to induce a calming effect. The peak effect for rectal administration occurs at 1.5 hours. The negative attributes of diazepam for pediatric sedation are that it has a long half-life, active metabolites, and erratic absorption. Additionally, it causes pain with injection.
IV and IM dosing depend on age. Dosing is not established in neonates younger than 30 days. In children aged 30 days or older, IV or IM dosing is 0.25 mg/kg/dose; the agent should be administered over 3 minutes to avoid respiratory depression. The dose may be repeated after 15-30 minutes, with care taken not to exceed 10 mg/dose.
Rectal gel dosing also depends on age. Dosing is not established in children younger than 2 years. In children aged 2-5 years, the rectal dose is 0.5 mg/kg/dose. In children aged 6-11 years, rectal dosing is 0.3 mg/kg. In children aged 12 years and older, rectal dosing is 0.2 mg/kg.
Barbiturates
Barbiturates elicit action at GABA receptors and hyperpolarize the nerve cell membrane via chlorine channels. They produce sedation and amnesia and reduce anxiety, but they have no analgesic effects. These medications produce a reproducible dose-response effect, based on weight. Additionally, they have neuroprotective properties (derived from their ability to lower intracranial pressure [ICP]) and anticonvulsant properties. Disadvantages include hypotension, hypoventilation, and apnea.
Pentobarbital
Pentobarbital is widely used for procedural sedation. It is a short-acting barbiturate with sedative, hypnotic, and anticonvulsant properties. When administered IV, pentobarbital has a prompt onset of sedation (within 3-5 minutes), and its duration of action is 15-45 min. When administered IM, it produces sedation in 10-20 minutes and has a duration of action of 1-2 hours.
Dosing in children is 2-5 mg/kg/dose by slow IV push, not to exceed 100 mg/dose. IM dosing is 2-6 mg/kg/dose, not to exceed 100 mg/dose.
Methohexital
Methohexital is an ultrashort-acting barbiturate. Its onset of action occurs in less than 1 minute, and its duration of action is about 10 minutes.
Induction dosing is 0.75-1 mg/kg/dose IV. Maintenance dosing is 0.5 mg/kg/dose IV every 2-3 minutes.
Miscellaneous agents
Induction/hypnotic agents that provide rapid loss of consciousness include nitrous oxide, ketamine, and propofol. Ketamine also provides analgesia and amnestic effects. Etomidate, an ultrashort-acting sedative, is used frequently in adults for rapid sequence induction. It appears to have neuroprotective properties and minimal cardiovascular effects. Although some studies suggest that etomidate may be efficacious in pediatric patients, the utility of this agent in this population has not been fully established.
A small study compared the effects of etomidate plus fentanyl with those of ketamine plus midazolam during orthopedic reductions in a pediatric ED. [15] The results indicated that the incidence of adverse reactions to ketamine, including emergence phenomenon, may be lower than was previously thought.
Nitrous oxide
Nitrous oxide elicits anxiolysis, amnesia, and mild-to-moderate analgesia. However, its analgesic property is variable, typically requiring additional analgesic agents. [16] Nitrous oxide has little effect on the cardiovascular and respiratory systems, with only minimal effect on the airway reflex. The peak onset of action occurs within 30-60 seconds, the maximum effect within 5 minutes. Effects are rapidly lost once inhalation ceases, and recovery occurs within 5 minutes.
A 1:1 mixture of oxygen and nitrous oxide is administered as an inhalant via a handheld mask or mouthpiece. Typically, patients are to maintain the seal to ensure adequate inhalation; once sedation is approached, the patient will lose the seal and allow the mask or mouthpiece to fall.
Ketamine
Ketamine is a dissociative agent that induces catalepsy. It exhibits sedative, analgesic, and amnestic properties. This agent is related to phencyclidine (PCP) and has shown a history of efficacy. It preserves the airway reflexes and has minimal effect on the respiratory drive. Ketamine has bronchodilatory effects and is especially effective with bronchospasms. In addition, it has a good safety profile in children.
After IV administration of ketamine, peak onset of action occurs within 1 minute, and the duration of action is about 10-15 minutes. After IM administration, the peak onset of action occurs in about 5-10 minutes, and the duration of action is 15-30 minutes.
Because of the risk of hypertension, dysphoria, and agitation, ketamine is rarely used in adults. In pediatric patients, ketamine may (rarely) cause laryngospasm. The reaction appears to be idiosyncratic and not to be a function of age, dose, coadministration of anticholinergics, or other clinical variables. [17]
Dosing is 1-1.5 mg/kg by slow IV push (not to exceed 0.5 mg/kg/min). Additional doses may be administered at 0.5 mg/kg IV every 10-15 minutes, depending on the patient’s response and the duration of the procedure. Alternatively, ketamine may be given as 4 mg/kg IM, and additional doses of 2-4 mg/kg may be administered—again, depending on the patient’s response and the duration of the procedure.
A 2013 study demonstrated the benefit of performing sedation with a newly available combination of ketamine and propofol, known as ketofol, rather than with ketamine plus fentanyl for sedation. Switching to the newer combination resulted in a 14% decrease in adverse events overall, a 7% decrease in oxygen desaturation, and a 9% decrease in nausea and vomiting. The decrease in desaturation requiring positive pressure ventilation was 35%. [18]
A retrospective study examined the data that suggested an advantage of sedation with the combination of ketamine and propofol over ketamine alone or propofol alone. The study found that sedation with the combination of ketamine and propofol can be safely performed by a skilled emergency physician. [19] However, a prospective, multicenter, observational cohort study from 6 pediatric emergency departments that examined 6295 cases of pediatric sedation reported that the use of ketamine alone resulted in the lowest incidence of serious adverse events (17 [0.4%]) and significant interventions (37 [0.9%]) compared to just propofol or a combination of ketamine with propofol or fentanyl. [20]
Propofol
Propofol is a unique medication that has no relation to either of the usual sedative classes (ie, benzodiazepines and barbiturates). It is a purely sedative agent without any analgesic or amnestic properties.
Because of its high lipid solubility, propofol has a rapid onset of action (within 40 seconds). Its duration of action is 1-3 minutes, allowing swift emergence and recovery. Preliminary pediatric studies indicate that propofol is efficacious in terms of providing sedation and is easy to use.
Monitored anesthesia care (MAC) sedation dosing is 0.5-1 mg/kg by IV push, infused over 2 minutes initially. Maintenance dosing is 0.5-1 mg/kg IV every 3-5 minutes as needed or, alternatively, 50-150 µg/kg/min by continuous IV infusion.
Initially, propofol was used as an induction agent in general anesthesia. However, it has also been used for sedation in intubated patients in intensive care units (ICUs) and in patients undergoing diagnostic imaging studies, such as computed tomography (CT) and magnetic resonance imaging (MRI). Propofol is now an important sedation agent among ED physicians. Controversy exists among anesthesiologists and ED physicians regarding optimal use of this drug in patient management. [21]
Procedural sedation policy governing propofol use is typically under the direction of the anesthesiology department. Anesthesiologists have often opposed ED use of propofol because of the potential risk of deep sedation (leading to general anesthesia) and hypoxic/respiratory depression. However, emergency medicine physicians have the technical skills for rapid-sequence intubation and advanced airway management. The ED practice environment is tailor-made for propofol use in a brief controlled setting for procedural sedation. [22]
Increasing numbers of studies describe propofol use in the ED. Patel et al described how propofol provided effective moderate sedation for procedures lasting 30 minutes or longer and outlined effective dosing regimens in different age groups. [23] In this study, the sedation was performed by a pediatric sedation service staffed by nonanesthesiologists.
Dexmedetomidine
In December 2022, the FDA approved dexmedetomidine for sedation in nonintubated pediatric patients aged 1 month to 18 years prior to and/or during surgical and other procedures. [24] Dexmedetomidine is a medication that has the ability to provide sedation without causing respiratory depression. It resembles clonidine in that it is an alpha2 -adrenergic receptor agonist. It has been administered to adults by IM injection for perioperative anxiolysis and sedation. Evidence suggests that dexmedetomidine may be useful for procedural sedation in children. [25, 26] A report from AAP’s Pediatric Sedation Research Consortium reviewed use of dexmedetomidine for procedural sedation in 13,072 pediatric patients, concluding it as a high success rate with minimal side effects. [27]
Adjuvant Therapies
Nonpharmacologic approaches to sedating children are gaining increasing popularity. Some of these adjuvant techniques require training, but once learned, they can easily be integrated into wound care or postprocedural pain management.
To a large extent, adjuvant therapy involves distracting the patient. This may require the use of coping skills to focus attention away from the procedure. Physical objects that appeal to children can provide visual or auditory distractions. Role-playing, such as pretending to perform a procedure, may help alleviate a child’s fear. Guided imagery, hypnosis, and distractions can be powerful adjuvants to sedation in children and adults.
Also helpful is allowing family members to remain with the child during the painful procedure. Having a family member at the bedside may decrease distress for both the child and the parent. Tell anxious children to close their eyes and pretend that they are in their favorite place. Involving parents by having them tell a story or distract the child goes a long way toward reducing anxiety.
Postprocedural Care
After the completion of the procedure, it is important to keep recording vital signs until the patient responds appropriately to a voice or gentle stimulation. Sedation is stimulus-dependent; accordingly, when the procedure is completed, the child is likely to become more sedated than he or she was during the procedure. This can lead to hypoventilation and hypoxia if the child is not closely monitored.
In general, discharge criteria should include the following:
-
The child’s vital signs should be within 15% of admission readings (either above or below)
-
The child should be ambulatory as appropriate for his or her age, without assistance
-
The child should be able to ingest and retain oral fluids
Some agents are associated with specific aftercare needs. For example, ketamine may cause ataxia for 12-24 hours, and the child’s activities should be restricted during this period to prevent further injury.
Questions & Answers
Overview
What are the benefits of pediatric sedation administered in the emergency department (ED)?
Which emergency department (ED) diagnostic procedures require pediatric sedation?
Which emergency department (ED) therapeutic procedures require pediatric sedation?
Which safety precautions are taken prior to administering pediatric sedation?
What is the focus of the clinical history prior to pediatric sedation?
What is the focus of the physical exam prior to administering pediatric sedation?
What monitoring is provided during pediatric sedation?
Which medications are used for pediatric procedural sedation?
Which reversal agents are used in pediatric procedural sedation?
How are children monitored following pediatric sedation?
What are the discharge criteria following pediatric sedation?
What are the guidelines on pediatric sedation?
What is the sedation continuum?
How is pediatric procedural sedation defined?
What are the myths regarding pediatric pain management?
What do clinicians need to know about pediatric pain management?
Which factors affect how a child responds to a painful procedure?
What are the indications for the use of pediatric sedation in the emergency department (ED)?
Which factors affect the selection of pediatric sedation agents and administration route?
How is dosing determined for pediatric sedation?
What steps should be taken to reduce the risks of pediatric sedation?
What is included in the preprocedural evaluation of children prior to sedation?
What is the ASA physical status classification for pediatric patients undergoing sedation?
How are children monitored during procedural sedation?
What is the role of opioid analgesic agents in pediatric sedation?
What is the role of morphine in pediatric sedation?
What is the role of fentanyl in pediatric sedation?
What is the role of benzodiazepines in pediatric sedation?
What is the role of midazolam in pediatric sedation?
What is the role of diazepam in pediatric sedation?
What is the role of barbiturates in pediatric sedation?
What is the role of pentobarbital in pediatric sedation?
What is the role of methohexital in pediatric sedation?
What is the role of thiopental in pediatric sedation?
What is the role of induction/hypnotic agents in pediatric sedation?
What is the role of nitrous oxide in pediatric sedation?
What is the role of ketamine in pediatric sedation?
What is the role of propofol in pediatric sedation?
What is the role of dexmedetomidine in pediatric sedation?
What are the nonpharmacologic adjuvant approaches to pediatric sedation?
What is included in the postprocedural care following pediatric sedation?
-
Mallampati classification.
Tables
What would you like to print?
- Practice Essentials
- Overview
- Sedation Terminology
- Factors Affecting Pain Response
- Indications for Emergency Department Sedation
- Preparing for Sedation
- Preprocedural Evaluation
- Intraprocedural Monitoring
- Sedative Medications
- Adjuvant Therapies
- Postprocedural Care
- Questions & Answers
- Show All
- Tables
- References