Overview
Foci of infection in the oral cavity and their relationship with the overall health of the human body have been long studied among healthcare practitioners throughout history. The father of medicine, Hippocrates, mentions an association between resolution of arthritis and extraction of a decayed tooth back near 400 BCE. Research on the relationship between oral health and systemic diseases gained rapid acceleration after the death of President Theodore Roosevelt in 1919 from odontogenic sepsis. [1]
The oral cavity, being considered as "the intersection of dentistry and medicine" and "the window to general health", contains some of the most varied and vast flora in the human body and is the main entrance for two systems vital to human function and physiology, the gastrointestinal and respiratory systems. [2] Therefore, specific infections in the oral cavity may create foci of infection that may affect systemic health. [3]
More than 500 species of bacteria, viruses, fungi, and protozoa are identified in the oral cavity. [2] A variety of organisms in the microenvironment of the oral cavity adhere to the teeth, the gingival sulcus, the tongue, and the buccal mucosa. When a local or systemic disease process or concomitant use of medications alters this overall pattern, atypical organisms begin to predominate and some normal organisms with a benign nature may become pathogenic. The microenvironment of the oral cavity may change according to the age of the patient, eruption or loss of teeth, or active other conditions (eg, caries, periodontal disease). Systemic changes, such as pregnancy or drug intake, may also alter the number and proportion of the oral flora. These changes are due to alterations in the flow and composition of salivary fluid and in the levels and activity of defense components (eg, immunoglobulins, cytokines) in the saliva. [4]
One of the most common chronic bacterial oral infections, periodontitis, affects the supporting structures of the teeth. The host response to this infection is an important factor in determining the extent and severity of the disease. Systemic conditions may modify the extent of periodontitis principally through their effects on normal immune and inflammatory mechanisms.
Oral foci of infection have significant impact on the systemic well-being of an individual and are associated with chronic conditions such as cardiovascular diseases, diabetes mellitus, kidney diseases, pulmonary diseases, or even pregnancy-related complications. [2, 5]
Increasing evidence indicates that oral microbiota participate in various systemic diseases. Most patients ignore or are unaware of the fact that poor oral health may have a serious impact on their overall health. Therefore, maintaining a good oral hygiene through patient education and providing the best oral care must be the primary goal for both general healthcare and oral-care physicians in order to prevent major consequences.
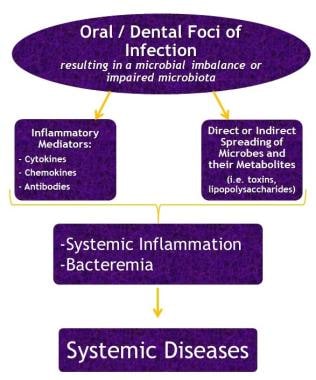
See the flowchart below.
Cardiovascular and Cerebrovascular Disease
The most common cause of mortality worldwide is cerebrovascular disease (CVD), which includes coronary heart disease (CHD), congestive heart failure, CVD and stroke, peripheral artery diseases, carotid artery diseases, and aortoiliac disease. [5] The primary cause of both CHD and CVD is atherosclerosis, which is defined as the pathologic narrowing of arteries due to the deposition of cholesterol and its products. [2] Many systematic reviews and a scientific statement from the American Heart Association state the possible role of poor oral health on the elevated risk of CHD. [6] Periodontal bacterial infections may permit pathogens to colonize distant sites; therefore, the association between certain periodontal pathogens and coronary artery disease or stroke is gaining more importance. This association is suggested by the pathogen's ability to predispose the arteries for atherosclerosis. Periodontal pathogens have been found in carotid and coronary atherosclerotic plaques. [5, 7] Although the underlying mechanisms are complex, the chronic inflammatory state and microbial burden in people with periodontal disease may predispose them to cardiovascular diseases in ways proposed for other infections. [8]
Etiology and pathogenesis
Several studies reveal the following three mechanisms possibly responsible for cardiovascular diseases due to oral pathogens [7] :
-
Intracellular invasion of arteries by periodontal pathogens
-
Increased responsiveness of the immune response to these pathogens with possible involvement of proinflammatory bacteria
-
Cross-reactivity of antibacterial heat-shock protein antibodies with human heat-shock proteins.
Host inflammatory responses are activated in patients with periodontal disease and poor oral hygiene, since they experience frequent episodes of severe gingival inflammation and bacteremia. Proinflammatory cytokines, namely C-reactive protein (CRP), tumor Necrosis factor-α, interleukin 1β, and Interleukin 6, are triggered as a result of this chronic inflammatory condition. These proinflammatory cytokines, along with bacteremia, not only stimulate the process of atherogenesis but also increase the susceptibility of the vascular endothelium to injury. This state of predisposition functions as a precursor to atherogenesis. [2]
An elevated level of CRP is considered a risk factor for cardiovascular disease. The CRP level may fluctuate according to the variety of inflammatory stimuli and is considered useful as a method of monitoring inflammatory changes in the arteries that may result in acute thrombosis or clinical conditions such as myocardial infarction. Consequently, periodontal disease, which is associated with elevated serum CRP, may be blamed for triggering a complement-mediated inflammation that contributes to atheroma formation or other cardiovascular events. [5, 9]
Shared genetic risk factors between coronary artery disease and periodontitis have been studied and classified in the literature. All known shared risk genes for coronary artery disease and periodontitis have been identified from the members of transforming growth factor-β signaling. [10]
Bacterial virulence factors such as endotoxin lipopolysaccharides are present in the subgingival plaque of patients with severe periodontal disease. Lipopolysaccharides and other bacterial components also activate inflammatory cytokines and thus play a role in cardiovascular diseases through either direct or indirect mechanisms. Lipopolysaccharides and inflammatory cytokines increase the expression of leukocyte adhesion molecules by endothelial cells. These adhesion molecules are associated with atheroma formation. [5]
On a microbiological perspective, streptococci of the viridans group may induce platelet aggregation and possibly thrombus formation as a result of dental bacteremia. The clumps of thrombus contribute to atherosclerotic CVD, which may lead to strokes or and transient ischemic attacks. [2] Porphyromonas gingivalis has been identified in coronary or femoral arteries as the most abundant species in patients with atherosclerotic CVD who underwent arterial bypass surgery. P gingivalis is reported to represent nearly 80% of all bacterial species in the arteries of these patients. [7]
Massive colonization of P gingivalis in also clinically healthy arteries supports the concept that P gingivalis possesses unique properties to invade the arterial walls to survive intracellularly and escape the immune system. [7] However, it is still unclear whether the bacterial colonization of healthy tissues occurs before or after the onset of atherosclerotic disease. Other unclear factors are the duration in which bacteria remain alive within tissues and the type of species that act as bystanders or contributors to the development of atherosclerosis in the initial stages of the disease. [7]
Following P gingivalis,Enterococcus faecalis, Finegoldia magna, and Pseudomonas aeruginosa, are detected with a relative abundance of nearly 3% compared with all remaining species, detected at less than 1%. E faecalis is associated with infective endocarditis (see next section, Bacterial Endocarditis). [11, 12] F magna is a frequent pathogen involved in postoperative and prosthetic implant–related infections and mediastinitis following coronary artery bypass. On the other hand, F magna is rarely detected in atherosclerotic plaques from carotid arteries. [7]
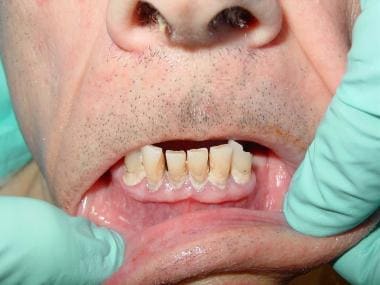
See the images below.
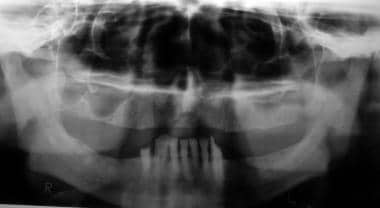 Radiographically evident severe alveolar bone loss and periodontitis in a 72-year-old man with severe periodontal disease.
Radiographically evident severe alveolar bone loss and periodontitis in a 72-year-old man with severe periodontal disease.
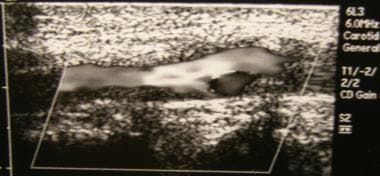 Carotid duplex ultrasonogram exhibits severe atherosclerosis/stenosis of the left carotid artery in a 72-year-old man with severe periodontal disease.
Carotid duplex ultrasonogram exhibits severe atherosclerosis/stenosis of the left carotid artery in a 72-year-old man with severe periodontal disease.
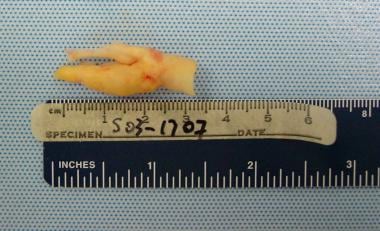 Gross pathologic specimen shows carotid plaque as cause of stenosis in a 72-year-old man with severe periodontal disease.
Gross pathologic specimen shows carotid plaque as cause of stenosis in a 72-year-old man with severe periodontal disease.
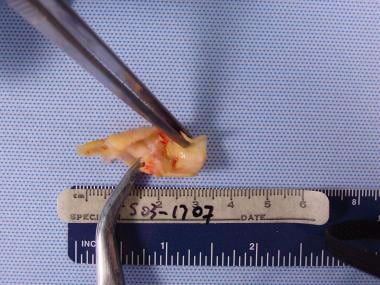 Dissected pathologic carotid plaque with bacterial contents similar to periodontal plaque surrounding existing dentition in a 72-year-old man with severe periodontal disease.
Dissected pathologic carotid plaque with bacterial contents similar to periodontal plaque surrounding existing dentition in a 72-year-old man with severe periodontal disease.
Epidemiology
The association between atherosclerotic vascular disease and poor oral health has gained more importance over the past two decades. [13] Studies reveal that patients with a history of myocardial infarction or cerebrovascular attacks have worse oral health when compared with control groups. [2] A 2020 systematic review and meta-analysis involving 19 studies and 640,446 subjects reported that periodontitis is significantly associated with a greater risk of all-cause mortality and deaths due to both cardiovascular and cerebrovascular diseases, as well as CHD. [14]
Overall, there is an increased risk of atherosclerotic vascular disease in patients with periodontitis compared with that in patients without the disease. Several clinical parameters of periodontitis, such as periodontal probing depth, clinical loss of attachment, or radiographic bone loss, have been linked to an increased risk of atherosclerotic vascular disease, independent of established risk factors. [13] Specifically, there is an increased risk in males and younger individuals. [2, 13]
The relative risk of periodontal patients for mortality was 2.58 for CHD and 3.11 for CVD with respect to a population with periodontal health. [15]
Dental considerations
Patients with CVD should be assessed for any risk factors and foci of infection. Premedication may be considered to alleviate anxiety. Effective analgesia is important to reduce stress-related outcomes in these patients. Procedure time should be kept to a minimum, and treatment should be terminated early if the patient becomes overly anxious. In addition, it is best to give early and short morning appointments. A thorough evaluation should include a record of the current medications patients are taking, and any potential drug interactions or adverse effects should be clearly noted. Collaboration with the patient’s cardiologist or general healthcare provider regarding any anticoagulant or antiaggregant therapy should be delicately handled. [16]
According to the discussions on the etiology and pathogenesis of atheroma in patients with severe periodontal disease, the goals of treatment are to treat hypercholesterolemia and lower low-density lipoprotein levels via any of the new statin medications in conjunction with a low-fat, low-cholesterol diet. Maintaining good oral hygiene and eliminating the oral foci of infection and bacterial diseases are additional methods used to eliminate the oral cause of atheroma formation. [17]
Although some studies emphasize that if a patient is both diabetic and has a severe periodontal condition, there is a higher risk of cardiorenal mortality compared with those with better oral health, there are several others that do not support the effect of periodontal treatment on cardiovascular diseases, especially when other additional comorbidities are involved such as diabetes mellitus. [18, 19, 20] A meta-analysis also failed to prove the efficacy of periodontal therapy on the CVD burden in the long term. [21]
Although the evidence is controversial, periodontal therapy does trigger a short-term inflammatory response and reduces some degree of systemic inflammation and improves endothelial function. However, the evidence is limited to truly prove that these acute and chronic changes will either increase or reduce the CVD burden of individuals with periodontitis in the long term. [21] Moreover, there is an insufficient amount of interventional studies to reveal the preventive effects of periodontal treatment on the future incidence of cardiovascular disease. [13]
Bacterial Endocarditis
Bacterial endocarditis (BE) or infective endocarditis is defined as a bacterial infection of the endocardium. It is considered highly morbid and mortal, particularly in compromised patients. Patients with prosthetic heart valves, those with congenital or structural heart disease, or those who abuse intravenous drugs are considered high-risk patients. [22, 23] The most predisposed to BE are those with congenital heart defects and especially patients with valvular heart disease of rheumatic origin. [24]
BE mainly damages the heart and causes valve destruction or heart failure. Cardiac symptoms manifest as changing murmurs showing heart damage, infection, or embolic damage in various organs, especially the kidneys. Embolic damage may also have other systemic consequences ranging from a loss of the peripheral pulse to sudden death due to stroke. Other symptoms may include light pigmentation of the skin, joint pain, or hepatosplenomegaly. BE is diagnosed via four factors: changing murmurs, electrocardiography (diagnosis of abnormal rhythms), echocardiography, and evaluation of valve and heart function and blood culture. [25]
BE tends to develop on cardiac valves that were previously damaged. The mitral valve is the most frequently affected, followed by the aortic and, in rare occasions, the pulmonary valve. [25] It most commonly involves the heart valves (either native or prosthetic), but it may also occur at the site of a septal defect, on the chordae tendineae, or on the mural endocardium. [26]
Etiology and pathogenesis
Oral infections and oral bacterial microbiota play a key role in BE. Bacteremia may be induced not only after invasive procedures, but also after routine oral hygiene activities, such as teeth brushing and flossing, or even during chewing. Interestingly, American Heart Association states that the intensity of bacteremia following a tooth extraction is similar to that induced by tooth brushing or flossing. [27] Although these are temporary episodes of bacteremia with a low bacterial load, they sometimes may last up to 1 hour. [22] Still, the magnitude or duration of bacteremia associated with an oral/dental origin is controversial. [24]
The complex interaction between the bloodstream pathogen with matrix molecules and platelets at sites of endocardial cell damage is considered the main mechanism in the development of BE. [24] In most cases, the initial steps of BE begin in a mass formed mainly by platelets and fibrin that originally is microbe-free but susceptible to be colonized by microorganisms. Nevertheless, most bacteremias are transient and self-limited and do not have systemic complications. [22]
Staphylococcus, Streptococcus, and Enterococcus species are responsible for 90% of BE cases. Oral flora is a host to oral streptococci, which is a part of the viridans group (Streptococcusmutans and Streptococcus sanguis). These microorganisms, as part of the dental plaque, may enter the bloodstream and cause bacteremia through daily habits like chewing or tooth brushing. Dental extraction or other dental procedures also may cause bacteremia. [25]
There is also a group of organisms called the HACEK group, which consists of Haemophilus species, Aggregatibacter species, Cardiobacteriumhominis, Eikenella corrodens, and Kingella species. These are part of the normal human oral flora and may be responsible for approximately 3% of valvular BE cases. Although HACEK infections are not common, they may have extremely serious consequences. Still, they are usually successfully managed if the organism is identified early and treated appropriately. [28]
Epidemiology
BE used to be a disease prominent among young adults with rheumatic heart valve diseases; however, nowadays it is more common among elderly people, particularly after medical procedures. [22, 23] BE has an annual prevalence of 2-8 cases per 100,000 individuals. There is a much higher incidence in individuals aged 70-80 years. [22, 29] BE in valve disease patients is encountered statistically in 1 in 3,000 cases. BE is now rare amongst younger populations, with the exception of intravenous drug users. [25]
People with congenital heart disease or those who have undergone valvular operation are almost equally likely to be affected by BE, but patients with a history of noncomplex myocardial infarction or noncomplex angioplasty, as well as patients with coronary bypass or cardiac pacemakers, are less at risk for BE. It has been estimated that 8-10% of endocarditis cases are related to oral infections. [25]
Dental considerations
BE is a fatal disease in 30% of the cases if no treatment is initiated. Routinely, intravenous antibiotic therapy (benzylpenicillin and gentamicin) is indicated for an extended treatment period. Cases of staphylococcal endocarditis are treated with vancomycin instead of penicillin. Severe cases, such as prosthetic valve endocarditis, may require replacement of the infected valve. [25]
Prophylaxis against BE with antibiotics is still debated. Some studies report that antibiotics administered before a dental procedure may reduce the frequency or duration of bacteremia, while others fail to support this statement. [24]
According to the American Heart Association, antibiotic prophylaxis is recommended for patients at the highest risk of adverse outcomes from BE. Dental procedures on gingival tissues or the periapical region of a tooth and procedures that perforate the oral mucosa require antibiotic prophylaxis. Antibiotic regimens for patients at high risk of BE undergoing dental procedures are shown in the Table below. Cephalosporins are not recommended for patients with a history of anaphylaxis, angioedema, or urticaria after taking penicillin or ampicillin. [23, 27]
Table. Prophylactic Antibiotic Regimen for Bacterial Endocarditis (Open Table in a new window)
| Agent | Dosage (Adult) | Dosage (Pediatric) | Route of Administration |
|---|---|---|---|
| Amoxicillin | 2 g | 50 mg/kg | Oral |
| Ampicillin | 2 g | 50 mg/kg | Intramuscular (IM) or intravenous (IV) |
| Cefazolin or Ceftriaxone | 1 g | 50 mg/kg | IM or IV |
| Cephalexin | 2 g | 50 mg/kg | Oral |
| Clindamycin | 600 mg | 20 mg/kg | Oral, IM, or IV |
| Azithromycin | 500 mg | 15 mg/kg | Oral |
| Clarithromycin | 500 mg | 15 mg/kg | Oral |
Diabetes Mellitus
Diabetes mellitus (DM) is defined as the disruption of glycemic control due to lack of insulin production (type 1 DM) or systemic insulin resistance (type 2 DM), which results in a prolonged exposure to hyperglycemia. Prolonged hyperglycemia is the main cause of DM-related complications, and it negatively affects the heart, eyes, kidneys, and peripheral nerves. Moreover, oral infections are also suggested as one of the major complications of DM. [2, 30]
There is a bidirectional association between DM and oral health. This means that not only does hyperglycemia have a negative impact on oral health, but conversely, poor oral health has an adverse effect on glycemic control. [2] The severity and prevalence of several oral diseases are increased in diabetic patients and are worse in those with poorly controlled diabetes. Periodontitis is reported to exacerbate DM by decreasing glycemic control. [31]
Etiology and pathogenesis
Oral complications related to DM are due to hyperglycemia, which results in the production of advanced glycation end products. [2, 32] Advanced glycation end products are responsible from a systemic impact, consequently causing increased cytokine production, local inflammation, and loss of connective tissue. Local inflammation in the oral cavity has a systemic influence on glycemic control, which becomes more challenging as it also possesses a higher risk of infection. [2] DM patients are more prone to infections because immunologic functions including chemotaxis, phagocytosis, and microbicidal properties of polymorphonuclear neutrophils, macrophages, monocytes, and T lymphocytes are impaired. In addition to hyperglycemia and immune dysfunction, insulin resistance, dyslipidemia, and hypertension are other mechanisms responsible for the chronic complications of DM. [31]
Epidemiology
DM is considered as one of the leading causes of disability globally and is listed as the sixth leading cause of death. [33, 34] In 2014, 422 million people were diagnosed with diabetes, whereas this number was almost 100 million back in the 1980s. The global prevalence of DM among adults increased from 4.7% in 1980 to 8.5% in 2014. [33] Affecting approximately 10% of the global population, nearly 750 million people, severe periodontitis is considered the 11th most prevalent disease worldwide. [35, 36]
Dental considerations
DM is a risk factor for periodontitis; therefore, diabetic patients may potentially have higher rates of alveolar bone loss, abscess formation, and poor healing. Although diabetic patients have at least a threefold greater risk of periodontitis compared with healthy patients, studies show that patients with well-controlled diabetes have no increased risk of periodontitis compared with healthy individuals. [2] Dental caries, dry mouth, Candida-related lesions, and glossitis are also among possible oral complications of DM.
Several studies show that the prevalence of oral Candida infections in patients with both diabetes and periodontitis is approximately 52%. Poorly controlled type 2 DM patients are three times more prone to developing a periodontal disease than individuals without diabetes. Patients with a diagnosis of periodontitis are reported to have higher levels of HbA1c and fasting blood glucose than periodontally healthy individuals. Those with severe periodontitis have a significantly greater risk. [35] Appropriate periodontal treatment leads to a 0.4-0.6% improvement in HbA1c levels, meaning that periodontal treatment is associated with improved glycemic control. [20, 36]
Close collaboration between medical and dental clinical teams is necessary for the joint management of people with diabetes and periodontitis. [20] Overall, both management and prevention of not only systemic but also oral complications related to DM should be regarded as crucial aspects of modern diabetic care. Patients may benefit from an interdisciplinary approach of general healthcare physicians together with dental professionals to manage patients in the best possible way. [31]
Considering the bidirectional relationship between DM and oral health, and especially periodontal diseases, a combination of mechanical elimination of the biofilm and use of oral antibiotics, when indicated, has the greatest influence on both glycemic control and periodontal disease in patients with DM. [2, 32] Maintaining optimal oral health and patient education are of crucial importance and are a lifelong process for diabetic patients. The value of regular checkups should be emphasized, and the patient should be warned regarding the red flags that would require an immediate visit to an oral health professional.
Kidney Disease
Chronic kidney disease (CKD) is defined as reduced kidney function lasting longer than 3 months, and it is a major global health burden. In its early stages, CKD is frequently asymptomatic, but it presents with increased morbidity and mortality when kidney function becomes severely compromised. [37]
Patients with CKD commonly present with poor oral health and have a high prevalence for oral infections. [2, 38] This may lead to systemic inflammation, infection, protein wasting, and, as previously mentioned, atherosclerotic lesions. [2] Overall, poor oral health may contribute to increased mortality in CKD patients because of chronic low-level systemic inflammation caused by oral or dental infections. [38] Therefore, it is important to monitor and maintain the oral health status of patients with renal diseases, as well as patients who are considered as potential renal transplant candidates. [39]
Cardiovascular disease is the primary cause of morbidity and mortality in patients with CKD. Since periodontitis is considered to promote the progression of cardiovascular disease, cumulatively it may affect cardiovascular disease–related mortality in patients with CKD. [40]
A 10-year survival analysis of CKD patients reveals that the mortality rate increases from 32% to 41% when these patients present with periodontitis. [41] The results of this study have also been confirmed by a meta-analysis in 2017, which shows that periodontal disease is associated with an increased risk of death in CKD. [42]
Still, studies regarding an association between oral infections and CKD are highly heterogeneous, and this issue limits the significance of the overall results. [43]
Etiology and pathogenesis
Both CKD and chronic periodontitis affect the body with chronic inflammation and dysfunction of immune cells. [43]
There are several models to support the hypothesis that bacterial oral infections, specifically periodontitis, may contribute to the systemic inflammatory burden in end-stage renal disease patients. [44] Significantly higher serum CRP levels, which is an inflammatory biomarker, are detected in patients with poor oral health compared with healthy patients. This chronic low-grade systemic inflammation is considered a potential risk factor for CKD. However, although poor oral health may be one of the modifiable risk factors for CKD, it is often neglected in healthcare strategies. [37]
Hypertension, diabetes, and obesity are three major factors that lead to an increased prevalence of CKD. CKD patients reportedly have more severe oral diseases than the general adult population. Also, patients who die from CKD are more often diabetic and have fewer teeth than surviving patients. Oral infections, which have an adverse effect on glycemic control, should be highly prioritized especially in patients with diabetic CKD. [38]
In addition to end-stage renal disease, a form of glomerulonephritis called acute poststreptococcal glomerulonephritis (APGN) may also be triggered by either the deposition of bacterial toxins in the kidney or the deposition of nephrogenic streptococcal proteins in the glomerulus leading to an antibody-mediated response. [43]
Research suggests that the main pathogenesis is possibly not toxin-related but rather immunological. Several streptococcal proteins are identified (eg, nephritis strain–associated protein, endostreptosin, streptococcal M-protein) but the lack of specificity to APGN for these proteins is a major drawback in the identification of a specific causative agent. Consequently, alternative mechanisms, like autoimmunity, are proposed to have a role as well. [43]
Several researchers also suggest systemic inflammation, separately, due to both CKD and chronic periodontitis, may in fact lead to a bidirectional relationship between these two conditions. [45, 46]
Epidemiology
CKD has a worldwide prevalence of approximately 13.4%, which continues to rise annually. [43] In 2018, the World Health Organization published the global burden of kidney disease and reported that in 2015, 1.2 million people died from kidney failure, which indicated an increase of 32% since 2005. This number is believed to be an underestimation, considering the limited epidemiological data, the common lack of awareness, and the frequently poor access to laboratory services. [47] Oral periodontal diseases are shown to increase the mortality rate of CKD patients from 32% to 41%. [43]
The prevalence of periodontitis in patients with CKD varies considerably from 12.3% and 96.6%, which is attributed to the different definition of the diseases among studies. Prevalence of CKD in patients with periodontitis ranges from 7.2% to 21.6 %. [37]
Dental considerations
The stage of disease and clinical status are important factors to consider during the medical management of CKD patients. Patients with chronic renal failure may undergo dialysis, or, if they are suitable candidates, they may be considered for renal transplantation from cadavers or living donors. [48]
Cooperation between the oral healthcare physician and the nephrologist is critical in patients with CKD. Early evaluation of the oral health status of these patients may eliminate potential foci of infection due to an oral or dental origin. Preoperatively, all CKD patients should undergo a detailed oral assessment and obtain necessary dental treatment. [48, 49] This is crucial because the literature not only supports a positive association between CKD and oral periodontal diseases, but also emphasizes the positive effects of periodontal treatment on glomerular filtration rates. [50] Moreover, if a patient is both diabetic and has a severe periodontal condition, the incidences of macroalbuminuria and end-stage renal disease are increased twofold and threefold, respectively. [20]
Oral diseases or dental procedures may cause bacteremia, which may lead to morbidity and potential mortality in patients with renal failure or those on dialysis. Carious teeth, oral ulcers, plaque, and calculus may serve as points of entry for microorganisms into the bloodstream. Antibiotic prophylaxis before invasive dental procedures has been recommended by some authors, but this recommendation is contrary to guidelines from the British Society for Antimicrobial Chemotherapy. However, there are no clear guidelines for antibiotic prophylaxis regarding dental procedures in patients with CKD. [48, 49]
Good oral health is shown to lower the risk of oral infection, and, subsequently, the risk of septicemia, endocarditis, or enteritis at the site of vascular dialysis access. Patients should be motivated to maintain good oral care, and aggressive in-office oral health maintenance should be practiced to reduce the risk of dentally induced infections. [48]
It should be kept in mind that patients undergoing dialysis are at greater risks of infection by HIV and hepatitis types B and C. Such patients require liver function tests even before minor oral surgical interventions. [48]
Other considerations in patients undergoing dialysis are the risk of excessive bleeding, the risk of infection, and medication dosages. Appropriate coagulation tests and platelet counts should be checked before any oral surgical intervention. Usually, the day after dialysis is ideal for elective dental procedures because circulating toxins are eliminated, the intravascular volume is high, and the products of heparin metabolism are at an ideal state. The anticoagulant effects of heparin used during dialysis last only for 3-4 hours following an infusion and therefore does not trigger any bleeding abnormalities. [48, 51]
Respiratory Diseases
Respiratory diseases constitute a wide range of diseases involving both lungs and their anatomical parts. [5] One of the respiratory diseases, pneumonia, is commonly encountered among elderly persons, and others, such as lower respiratory tract infections, chronic obstructive pulmonary disease (COPD), and tuberculosis, are listed among the leading causes of early death. [34]
According to several studies, infections of oral cavity may be partly responsible for the development of respiratory diseases as potential foci of infection. [5] Although the exact pathogenesis of respiratory diseases due to poor oral health is unclear, it is anatomically known that the oral cavity is contiguous with the trachea and therefore may be a portal for respiratory pathogen colonization.
Patients on ventilators who have poor oral health reportedly have an increased risk of aspiration pneumonia. Also, the chances of acquiring pneumonia after a procedure involving endotracheal intubation is increased. [5, 52] In addition to pneumonia, COPD and lower respiratory tract infections are also associated with the aspiration of bacteria from the oral cavity into the lower respiratory tract. [2]
There is inconclusive evidence on a true association between respiratory diseases and oral health, but available data are trending toward a positive unidirectional relationship. Several high-quality studies reveal that improved oral health may have positive outcomes on systemic general condition, especially among institutionalized and ventilated patients. [2, 53]
Etiology and pathogenesis
Dental plaque (oral biofilm), residual roots, residues in oral mucous epithelium, and oral dryness as a result of impaired oral function may serve as potential oral foci of infection. [2] Lower airways may be contaminated via the following four possible routes:
-
Aspiration of oropharyngeal secretions, food, or gastric contents
-
Inhalation of infectious aerosols
-
Spread of infections from contiguous sites
-
Hematogenous spread from extrapulmonary sources of infection
Pathogens mainly enter the lungs through the aspiration of colonized secretions from the oropharynx into the upper airway, which can then be aspirated to the lower airway and adhere to the bronchial or alveolar epithelium. [54]
Bacteria from oral biofilm may be aspirated into the respiratory tract and may initiate an infectious respiratory condition. [55] An oral flora rich in oral pathogens such as Staphylococcus aureus, P aeruginosa, P gingivalis, or Aggregatibacter actinomycetemcomitans may bring about an aspiration of bacterial foci from the oral cavity into the lungs, increasing the risk of respiratory conditions. [2, 5]
Epidemiology
Pulmonary diseases consist of a wide group of conditions but COPD, asthma, acute lower respiratory tract infections, tuberculosis, and lung cancer are considered the five major respiratory condtions. [56]
COPD affects more than 200 million people globally. COPD was been ranked the seventh leading cause of early death in 2017. Lower respiratory tract infections are ranked higher on the list, as the fourth leading cause of early death. [34]
Dental considerations
Over the recent decades, there has been increasing debate over the relationship between poor oral health and respiratory disease. [57]
The literature supports an increased risk of pneumonia when patients have signs of cariogenic and periodontal pathogens in saliva, dental plaque, and dental decay. Interestingly, a previous history of respiratory tract infection has been reported in patients with higher plaque scores. One systematic review also emphasizes the importance of oral healthcare, stating that reducing the colonies of respiratory pathogens in the oral cavity decreases both mortality and morbidity. [54]
Although there is a reasonable amount of evidence to support an association between pneumonia and oral health, there is much poorer support to associate COPD and oral health. [57] On the other hand, there is substantial evidence that oropharyngeal decontamination with different antimicrobial interventions reduces the progression or occurrence of respiratory diseases. [54, 58]
Oral hygiene and frequent professional oral healthcare should be considered necessary for reducing the occurrence of respiratory conditions, especially among high-risk elderly adults living in nursing homes and those in intensive care units. In this group of patients, particularly if they are dentate, the risk of respiratory diseases may be prevented by oral hygiene interventions and frequent professional oral healthcare. [54]
Conclusion
Both the medical and dental healthcare professionals aim to optimize patients’ health and reduce the rates of morbidity and mortality.
Cardiovascular and cerebrovascular diseases, diabetes mellitus, kidney disease, and respiratory disease have been well studied for their possible relationship to oral health. Evidence-based medicine shows that oral infections may serve as possible contributory factors for these systemic diseases, as well as having an effect in disease progression. On the other hand, the results from studies on the effects of treatment of oral disease on systemic conditions seem controversial.
In the healthcare system, it is better to prevent than treat a condition, and that is why physicians should increase collaboration. Most patients may be unaware of the fact that a problem in their oral cavity may have a significant influence on their systemic health, and managing any coexisting condition may be enough to prevent a significant or even possibly life-threatening medical outcome.
Still, more research is required in this area to confirm a stronger association between oral health and systemic conditions.
-
A 72-year-old man with severe periodontal disease.
-
Radiographically evident severe alveolar bone loss and periodontitis in a 72-year-old man with severe periodontal disease.
-
Carotid duplex ultrasonogram exhibits severe atherosclerosis/stenosis of the left carotid artery in a 72-year-old man with severe periodontal disease.
-
Status post left carotid endarterectomy in a 72-year-old man with severe periodontal disease.
-
Gross pathologic specimen shows carotid plaque as cause of stenosis in a 72-year-old man with severe periodontal disease.
-
Dissected pathologic carotid plaque with bacterial contents similar to periodontal plaque surrounding existing dentition in a 72-year-old man with severe periodontal disease.
-
Flowchart showing the possible mechanisms linking oral/dental infections to systemic diseases.