Practice Essentials
Penile tumors present a difficult diagnostic and therapeutic issue, mainly because of their psychological implications. The diagnosis may be delayed because many patients tend to disregard early asymptomatic lesions and often seek medical attention at an advanced stage when a conservative surgical approach is no longer feasible. [1]
Among malignant neoplasms of the penis, squamous cell carcinoma (SCC) is the most common. [2, 3, 4] Verrucous carcinoma, also called Buschke-Löwenstein tumor, is a well-differentiated variant of SCC worthy of special recognition. [5, 6, 7, 8]
Primary SCC may occur at any anatomic site on the penis. It most often occurs on the glans, although it may also develop on the prepuce, both the glans and the prepuce, the coronal sulcus, and the shaft. Invasion of the shaft by a tumor originating from more distant sites may also be observed. [9] A rare case of lung SCC with metastasis to the penis in a 74-year-old man with an extensive smoking history was reported by Miura and Yamamoto. [10]
The treatment of penile SCC varies according to the clinical stage. Treatment includes radiation therapy, medical therapy (local and systemic), and surgery, alone or in combination. Laser therapy is also used. Because of the generally limited experience with SCC of the penis, considerable controversy exists as to the best form of treatment, specifically treatment for regional lymph nodes. [4, 11]
SCC of the prostate is a rare malignant epithelial neoplasm arising in the prostate, with squamous differentiation of the neoplastic cells. [12, 13] The time course for the appearance of squamous differentiation in the carcinoma varies from 3 months to many years (up to 9 years) after therapy.
Grossly, the tumors can be large, measuring up to 6.5 cm in greatest dimension. Cut surfaces are remarkable for a solid, firm, whitish-yellow, white-gray, to gray-tan mass. Central extension, with compression of the prostatic urethra, and local invasion into the bladder, rectum, and seminal vesicle may occur.
Also see Urethral Cancer.
Pathophysiology of Penile SCC
The cause of penile squamous cell carcinoma (SCC) is unclear, although human papillomavirus (HPV) appears to play a major role in many cases. In situ carcinomas may progress to invasive lesions. Other associations considered to play a role in the development of penile SCC include pre-existing dermatoses, lack of circumcision, and other factors, including environmental exposures.
A systematic review by Ribera-Cortada et al in 2021 noted that the most common mutations in cases of penile SCC involved the genes TP53, CDKN2A, FAT1, NOTCH-1, and PIK3CA. There were also additions in the genes MYC and EGFR, and amplifications were seen in HPV integration loci. The authors also noted that Hippo, Notch, and RTK-RAS pathways are often deregulated in penile SCC patients. [14]
In situ carcinomas
In situ carcinomas include Bowen disease, erythroplasia of Queyrat, and bowenoid papulosis. If untreated, these conditions may evolve to invasive carcinomas. Bowen disease and erythroplasia of Queyrat share similar histologic appearance and biological behavior and therefore are now usually considered different aspects of a single preneoplastic disorder. They are also defined as penile intraepithelial neoplasia, whereas the abbreviation Tis is used in the tumor-node-metastasis (TNM) classification.
Classification of bowenoid papulosis is controversial. The disease is probably better considered as a separate entity, owing to its peculiar clinical features, significantly lower rate of malignant progression, and better outcome. [4]
Kim et al found mutations of TERT-p in 18 out of 37 (48.6%) penile SCCs, including all 3 of the in situ cases. These mutations were significantly more common in non-HPV penile SCC cases than in HPV-associated cases. Also, TERT-p mutation was associated with favorable clinical and pathologic features, such as lack of lymph node involvement or distant metastases, low histologic grade, and low mitotic activity. [15]
Bowen disease
Bowen disease is rare, with the most common occurrence in elderly white men. Bowen disease usually occurs on the shaft and appears as a solitary, dull-red plaque with areas of crusting and oozing. Ulceration or papillomatous growth suggests evolution to invasive SCC, which occurs in approximately 5% of patients. Approximately 37% of the invasive lesions are associated with regional lymph node metastases. [16]
Erythroplasia of Queyrat
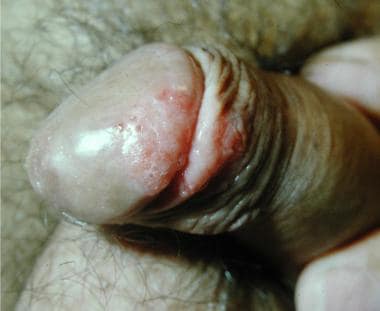
Erythroplasia of Queyrat is most common in elderly, uncircumcised white men. It appears as a solitary, sharply defined, bright-red, glistening, velvety, nontender, often-eroded plaque on the glans, the inner surface of the prepuce, or the coronal sulcus (see the image below).
Transformation into an invasive SCC is more common in erythroplasia of Queyrat than in Bowen disease, with a prevalence of 10-33%. Ulceration and/or papillary outgrowths are clinical signs of the disease. Approximately 20% of patients have regional lymph node metastases. [17, 18]
Bowenoid papulosis
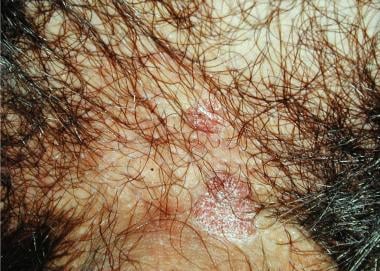
Bowenoid papulosis mainly occurs on the shaft in young circumcised men. It appears as multiple, small, slightly elevated, red-to-violet, slightly scaly or warty papules, which sometimes coalesce into large plaques (see the image below).
Extragenital involvement is exceedingly rare. [19] Bowenoid papulosis is an HPV infection with a characteristic bowenoid histology. Lesions may remain static, spontaneously regress, or progress to Bowen disease. Local recurrences after conservative excision and the onset of Bowen disease or SCC are reported in elderly and in immunocompromised patients. [2, 3, 20]
Preexisting dermatoses
Preexisting dermatoses include leukoplakia; HPV infections; penile lichen sclerosus; pseudoepitheliomatous, keratotic, and micaceous balanitis (PKMB); penile horn; and relapsing chronic balanitis of a different etiology (see below).
The true role of previous dermatoses, such as lichen planus, in the pathogenesis of SCC is still unsettled because the available data suggest that, in most cases, penile carcinoma arises de novo. Therefore, further investigations are needed to define the true incidence of the pre-existing conditions reported in association with penile SCC.
Leukoplakia
Leukoplakia is a clinical description of lesions appearing as slightly infiltrated, white, verrucous plaques on the glans or the prepuce related to squamous hyperplasia. [21] If ulceration, erosion, or fissuring is present, the likelihood of malignant degeneration is high because 10-20% of these lesions show dysplastic changes (basal atypias) upon histologic examination. [22, 23] Negative immunohistochemical staining for p53 and p16 may help to rule out in situ carcinoma in challenging cases. [24]
HPV infections
A role of HPV infections is established but not fully understood. [25, 26, 27, 28] Genital HPV infections mostly affect middle-aged, sexually active individuals and usually clinically appear as exophytic, fleshy, fibroepithelial proliferations involving the mucosal and cutaneous surfaces of the anogenital area (anogenital warts; see the image below).
However, subclinical HPV infections are common, especially in male sexual partners of females with in situ or invasive cervical carcinoma; they may be revealed by local application of an acetic acid solution. [29]
The evolution of HPV infections is related to the HPV type involved. HPV types 6 and 11 are usually detected in benign penile lesions, whereas oncogenic HPV types 16, 18, 31, and 33 are often found in in situ and invasive carcinomas. [30, 31] HPV sequences have also been found in local and distant metastases of penile SCC. [32, 33, 34]
The existence of 2 etiologically distinct types of penile SCC has been hypothesized: (1) a type with a viral cause and possible sexual transmission more likely to occur in younger individuals and (2) another type of unknown cause affecting older individuals. [4, 35, 36]
Penile verrucous carcinoma
Penile verrucous carcinoma has been linked to HPV infection; HPV types 6 and 11 are most frequently isolated. [2, 3, 4, 5, 6] Evolution of a verrucous carcinoma to SCC is possible, especially following irradiation. Invasive growth has been linked in some patients to the high-risk HPV types 16 and 18. [2, 3, 4, 5, 6]
Because HPV types 6 and 11 are usually isolated from benign lesions, other co-factors may be involved, including genomic alterations enhancing the oncogenic properties of these low-risk viruses; immunosuppression; co-infections by other viruses; the synergistic effect of environmental factors; poor hygiene; lack of circumcision; chronic irritation; [2, 3, 4] and previous dermatoses involving the genital area, such as genital lichen sclerosus, [35, 37] lichen planus, [38] or PKMB. [39]
Penile lichen sclerosus
Penile lichen sclerosus is a chronic, sclerosing, atrophic inflammatory condition that is more often observed in men, although boys may also be affected. Penile lichen sclerosus usually appears as white, atrophic papules on the glans and the prepuce that slowly coalesce into plaques, leading to meatal stenosis and/or phimosis (balanitis xerotica obliterans). Upon clinical examination, nodules or ulcerated plaques usually reveal malignant transformation (see the image below).
Although the likelihood of individual lesions of penile lichen sclerosus progressing into an invasive carcinoma is not predictable, this condition has a statistically significant risk of becoming SCC. Malignant changes have been observed in 9.3% of cases of penile lichen sclerosus in some series, and lichen sclerosus adjacent to a tumor has been detected in excision specimens from patients with penile SCC. [35, 37, 40, 41, 42, 43]
Studies have shown that lichen sclerosus is preferentially associated with HPV-negative, low-grade, differentiated, and highly keratinizing penile SCCs. [41, 44]
Polymerase chain reaction (PCR) testing has shown the presence of oncogenic HPVs in some cases of penile carcinoma arising on lichen sclerosus. [45] Patients with lichen sclerosus may be more susceptible to cancer development following infection by oncogenic HPV types than healthy subjects. Alternatively, HPV infection may hasten lichen sclerosus progression to cancer, resulting in a shorter lag-time. However, further investigations on the prevalence of HPV infection in patients with genital lichen sclerosus are needed to confirm these hypotheses. [4]
Pseudoepitheliomatous, keratotic, and micaceous balanitis
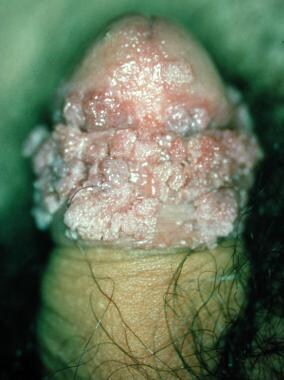
PKMB is an uncommon condition that occurs mainly in elderly men who are circumcised later in life. The lesion appears as a solitary, well-demarcated, hyperkeratotic plaque on the glans (see the image below).
Once considered benign, PKMB is now regarded as a tumor with a low-grade malignancy potential similar to that of verrucous carcinoma. [46] Degeneration into SCC clinically appears as a nodular lesion within the plaque.
Lichen planus
A study identified lichen planus in 9 of 35 specimens from men with penile SCC, suggesting a possible, although low, risk of SCC development in patients with this disorder. [47]
Penile horn
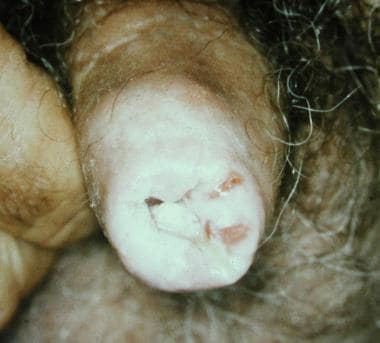
Penile horn is a rare condition that usually appears as a hard and conical keratotic mass with a bulging erythematous base that develops on the glans (see the image below).
These clinical features are not diagnostic because penile horns may microscopically reveal a benign epidermoid outgrowth (eg, warts); keratoacanthomas; or in situ, verrucous, or invasive carcinomas. [48, 49] Therefore, obtaining a biopsy sample of the base is mandatory, and clinical evolution and management depend on the underlying condition.
Relapsing chronic balanitis of a different etiology
Relapsing chronic balanitis of a different etiology (eg, bacterial, mycotic, viral) may be a pre-existing condition. According to some observations, chronic balanitis may, in time, lead to cancer development. [2, 3]
Factors resulting from the lack of circumcision
Epidemiologic data have demonstrated that penile SCC is exceedingly rare in men who are circumcised at birth. [50] The prophylactic effect of circumcision on penile carcinoma has been related to the lack of retained smegma. [51] A direct carcinogenic effect, however, is debatable; smegma retention may cause irritation and recurrent infections, leading to phimosis (reported in 25-75% of men with penile SCC).
One study also demonstrated an increased risk of HPV infection in uncircumcised men (19.6%) compared with circumcised men (5.5%). [52] On the other hand, phimosis itself is a risk factor for penile SCC development, [53] because it has been experimentally shown to induce histologic changes in the epithelium of the preputial sac.
Poor genital hygiene in uncircumcised males, even in the absence of phimosis, may also play a role, leading to the retention of smegma. This finding is confirmed by the lower incidence of penile SCC in countries with good hygienic standards. [2, 3, 4] However, a 2001 study failed to detect any correlation between personal hygiene habits (frequency and method of bathing and/or cleaning of the anogenital area) and risk of penile cancer development. [53]
Other factors associated with penile SCC
Several occasional factors that have been reported in association with penile SCC, as follows:
Prolonged exposure to different chemical compounds (eg, insecticides, fertilizers, styrene, acrylonitrile), together with poor hygiene practice, [54] traumas, [53, 55] or chronic irritation, [56] can be factors contributing to penile SCC.
Repeated management of urethral stricture with visual urethrotomy or urethral dilation [57] and genital piercing [58] have also been considered among the occasional causes of chronic penile inflammation predisposing to cancer development.
Late ritual circumcision followed by herbal treatments to control bleeding performed in the Southwestern Saudi region is associated with extensive scarring and may result in the development of invasive tumors proximally and dorsally located on the shaft. [59]
Buried penis secondary to obesity could also represent a risk factor, even in subjects circumcised at birth. [60]
Cigarette smoking has been found to show a clear-cut association with penile SCC; this association is related to the nicotine intake and is independent of phimosis or balanitis. Whether tobacco products concentrate in smegma has not been proven in humans. [53, 61]
Ultraviolet (UV) radiation may enhance the development of penile SCC, as observed in patients with psoriasis treated with psoralen plus UV-A or with UV-B. [62, 63]
Immune suppression, due to either transplantation or HIV infection, is associated with a greater risk of SCC development on the penis and on other skin sites. [64]
Reconstructive surgery for sex reversal can be a factor, although it is unclear whether the heterotopic penile skin within the neovagina is at an increased risk of cancer development. [65]
Pathophysiology of Prostatic SCC
Squamous cell carcinoma (SCC) of the prostate is a rare malignant epithelial neoplasm arising in the prostate, with squamous differentiation of the neoplastic cells. [12, 13] The time course for the appearance of squamous differentiation in the carcinoma varies from 3 months to many years (up to 9 years) after therapy.
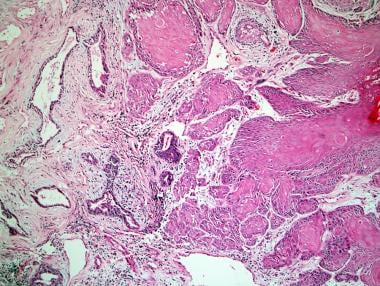
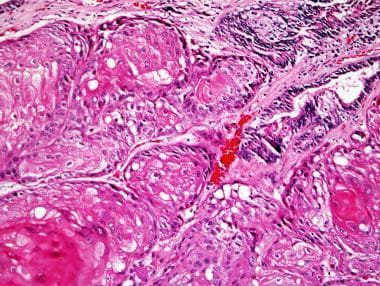
Microscopically, pure SCC shows infiltrating nests, strands, and sheets of polygonal cells with nuclear atypia, with squamous differentiation seen as individual cell keratinization, intercellular bridges, and/or keratin pearl formation (see the images below). These are readily apparent by light microscopy. In examples of adenosquamous carcinoma, glandular and squamous components can be distinct or exhibit direct transitions. In cases of adenosquamous carcinoma, the squamous component averages 40% of the tumor, with a range of 5-95%. [12]
Little is known of the molecular genetic abnormalities in these neoplasms. DNA content can be diploid or aneuploid. [66, 67] One case of adenosquamous carcinoma revealed p53 protein accumulation in both the squamous and glandular components. [68] These findings are not of diagnostic or prognostic value.
Prostatic SCC and adenosquamous carcinoma spread like pure glandular adenocarcinomas of the prostate, with growth along nerves; local extension into periprostatic soft tissue, bladder and seminal vesicles; and metastasis to lymph nodes and bone. In bone, the metastases are routinely osteolytic rather than the osteoblastic type that is characteristic of pure adenocarcinoma. [69] With widespread cancer, metastatic deposits have been detected in the glans penis, peritoneum, diaphragm, liver, and lung. There are also reported cases of prostatic adenocarcinoma transforming to metastatic SCC subsequent to androgen deprivation therapy. [70]
Many carcinomas in the prostate with squamous differentiation arise after radiation or hormonal forms of therapy. These treatment forms include diethylstilbestrol (DES), orchiectomy, luteinizing hormone-releasing hormone gene (LHRH) analogues, androgen receptor blockers, external beam radiation, and radioactive seed implantation.
The mechanism underlying the development of SCC or adenosquamous carcinoma of the prostate following radiation or androgen deprivation therapy is not known. Clearly, this pathway is generated in only very small minority of patients with adenocarcinoma of the prostate treated with hormonal therapy or radiotherapy.
Squamous metaplasia and human papillomavirus (HPV) infection do not appear to predispose to this development. Completely excluding a second squamous malignancy in these cases is not possible, although a more plausible explanation is clonal evolution/divergence of persistent carcinoma, secondary to the selective pressure of therapy.
Finally, other pathways for squamous prostatic carcinogenesis must exist because in some cases no therapy was administered before the diagnosis of squamous carcinoma, and prostatic SCC has been shown to occur in association with prostatic schistosomiasis. No chemical carcinogen has been associated with prostatic SCC.
Epidemiology
Penile SCC
Squamous cell carcinoma (SCC) accounts for at least 95% of all penile malignancies. [9] It represents approximately 2% of all cancers of the male genitalia and is found in 0.3-0.5% of the cancer-bearing male population. [71, 72] The overall incidence of primary malignant penile cancers (mean: 0.69 case per 100,000 population) has been reported as constantly decreasing from 1973 to 2002. [73] However, more recently, an increased incidence has been reported in Denmark and the United Kingdom. [74, 75]
The incidence of SCC varies in different geographic areas, ranging from 1% of all malignancies in male patients in the Western countries to 10-20% in some parts of Asia, Africa, and South America. Higher incidences are reported in tropical areas, such as Puerto Rico, Mexico, Paraguay, Venezuela, Vietnam, Ceylon, Thailand, China, Uganda, and parts of India. [2, 3, 4, 9] An incidence of 0.6 case per 100,000 population per year was recorded in the United Kingdom in 2007. [76]
Verrucous carcinoma represents 5-16% of all SCCs, and its prevalence varies from 5-24% among all penile malignancies. [5]
The age at onset of penile SCC is 20-90 years, with a peak around the sixth and seventh decades. [72, 77, 78] A few cases have been reported in children. [9] Penile verrucous carcinoma may occur at any age (18-88 y), but two thirds of all cases occur before age 50 years. [5, 6]
A racial predilection is unlikely, but statistical data are controversial because of possible confounders. In the United States, the highest prevalence of penile cancer is reported among Hispanics. [79] SCC is rare among some religious communities who practice circumcision, such as Jewish and Moslem groups and the Ibos of Nigeria. [2, 3]
Prostatic SCC
SCC of the prostate accounts for fewer than 1% of all prostate carcinomas. [12, 80] The largest case series reported is 33 cases. [12] The incidence depends on how the patient population is treated, as many carcinomas in the prostate with squamous differentiation arise after radiation or hormonal forms of therapy.
The incidence of adenosquamous carcinoma is lower than that of pure SCC, with only about 20 cases of adenosquamous carcinoma reported. In addition to admixture with adenocarcinoma, a few patients have had prostatic SCCs mixed with urothelial carcinoma and adenocarcinoma [81] and urothelial carcinoma. [82]
Diagnosis of Urogenital SCC
The differential diagnosis for penile squamous cell carcinoma (SCC) includes the following:
-
Metastatic Carcinoma of the Skin
Differentials for prostatic SCC
For prostatic SCC, other disorders to consider include the following:
-
Rectal SCC
-
Squamous metaplasia of prostate
-
Urethral urothelial (transitional cell) carcinoma with squamous differentiation
-
Urinary bladder urothelial (transitional cell) carcinoma with squamous differentiation
Workup for Urogenital SCC
Laboratory studies
Hypercalcemia may rarely complicate a penile carcinoma. [83]
Immunohistochemical studies are of limited use in the diagnosis of squamous cell carcinoma (SCC) of the prostate. The malignant squamous cells are most often negative for prostate-specific antigen (PSA) and prostate-specific acid phosphatase (PSAP) immunostains, although they are positive in almost all cases for high molecular weight cytokeratins using antibody 34betaE12. [12] The glandular component of adenosquamous carcinoma is typically positive for PSA and PSAP.
Imaging studies
Imaging techniques recommended for a more accurate staging of neoplastic disease and for periodic follow-up of treated patients include ultrasonography, computed tomography (CT) scanning, and magnetic resonance imaging (MRI). [84, 85]
Ultrasonography has the advantages of low cost, noninvasiveness, easy availability, and reliability in assessing extension of the primary tumor and the lymph nodes. When performed by a skilled physician, it is extremely sensitive in predicting infiltration of cavernous bodies. It may also have a role in determining whether metastatic nodes are resectable. [86] However, ultrasound is not particularly sensitive for metastases of inguinal lymph nodes in the absence of pathologically enlarged lymph nodes. Thus, a negative inguinal ultrasound does not preclude the necessity of a diagnostic lymph node dissection. [87]
CT scanning is widely performed and may be used in the staging of clinically positive lymph nodes, but it is not recommended for clinically node-negative patients and is of limited help in the assessment of primary penile lesions. However, patients with more invasive tumors (T1b or greater) or who have palpable inguinal lymph nodes should have cross-sectional imaging of the pelvis, chest, and abdomen to evaluate for potential metastatic disease. [87]
MRI improves soft tissue contrast and provides multiplanar high-resolution images, yielding the greatest accuracy in the evaluation of both the penile primary tumor and the lymph nodes. [88, 89] However, it is less sensitive in assessing urethral invasion and has some contraindications (eg, presence of vascular stents, pacing devices, prosthetic implants). [90] On T1-weighted and T2-weighted images, penile SCC usually appear as infiltrating masses that are hypointense in relation to the corpora. The masses appear hyperintense relative to fascia and the tunica albuginea on T2-weighted images. Galgano et al assert that small field of view T2-weighted images are the most relevant for local T-staging and assessment of adjacent structure involvement. [87]
Other imaging methods include the following:
-
Reflectance confocal microscopy (RCM): It has been suggested as a complementary technique to monitor the efficacy of laser photodynamic therapy in the treatment of in situ carcinoma. [93]
-
Lymphangiography and cavernosography: These have fallen out of favor because of their invasiveness.
-
Single-photon emission computed tomography (SPECT/CT): Based on the use of a Tc99m-labeled nanocolloid as a radiotracer, it has shown to be useful for improving inguinal lymph node metastases detection. [103] Additionally, a 2021 study of 143 patients with penile SCC found that 18F-fluorodeoxyglucose PET/CT imaging demonstrated greater diagnostic accuracy than CT alone. [104]
Biopsy
Initial biopsy of the primary penile lesion is necessary to confirm the diagnosis and to assess the grade and the invasiveness of the tumor, although a 2004 study has shown that it may fail to correctly assess the histologic grade (30% of cases) and deepest point of tumor invasion (91% of cases) when used alone. [105] Biopsy usually consists of a 1-cm elliptical wedge excision centered on the margin of the lesion. The mucosal application of 1% toluidine blue may aid in the identification of the best area from which to obtain a biopsy specimen. [4]
Other tests
In the diagnosis of long-standing, ulcerating condylomata, the identification of human papillomavirus (HPV) DNA by using molecular hybridization techniques (eg, Southern blotting, dot blotting, in situ hybridization, PCR) may be a useful diagnostic adjunct because lesions harboring oncogenic HPVs are reported to be at high risk of malignant degeneration.
Histologic Findings in Urogenital SCC
The diagnosis of cancer is always a histologic diagnosis. Histopathologic examination is essential to establish the diagnosis and to provide information about the extension of the tumor in deeper tissues. [106, 107] Upon microscopy, squamous cell carcinoma (SCC) may have different histologic features according to the degree of differentiation.
Histologic grading of penile SCC
Broders has described a classification of 4 groups (grades I through IV) with increasing aspects of atypia. [16] On this regard, a possible interobserver discordance may occur. [108]
Grade I includes the following:
-
Cells well differentiated with keratinization
-
Prominent intercellular bridges
-
Keratin pearls
Grade II-III includes the following:
-
Greater nuclear atypia
-
Increased mitotic activity
-
Fewer keratin pearls
Grade IV includes the following:
-
Marked nuclear pleomorphism
-
Numerous mitoses
-
Necrosis
-
Lymphatic and perineural invasion
-
No keratin pearls
-
Deeply invasive
Low-grade (grades I-II), well-differentiated lesions show a thickened, hyperkeratotic, and papillomatous epidermis, with a downward, fingerlike projection of atypical squamous cells, which often appear as concentrically arranged nests of cells surrounding keratin accumulations (keratin pearls).
Epithelial cells show intact desmosomes and slight atypia, with enlarged and pleomorphic nuclei and 1 or more prominent nucleoli. Mitotic figures are present. Individual cells may become dyskeratotic, appearing deeply eosinophilic. In the dermis, a dense lymphocytic or mixed inflammatory infiltrate may be present. [16]
Poorly differentiated SCC (grades III-IV) shows little or no keratinization, increased nuclear pleomorphism and hyperchromasia, and deeper invasion, and it may have areas of necrosis or superinfection. [16]
Histologic subsets of penile SCC
Several histologic subsets of SCC with different growth patterns, HPV association, biologic behavior, and prognosis have been identified. [4, 21, 101, 109] These include, besides the usual keratinizing variant (the most common subtype, accounting for 48-65% of all cases) [110] ; the verruciform variants, including warty (7-10%), papillary (5-15%), and verrucous (3-8%) types [107] ; the basaloid (4-10%) [107, 110] ; the warty/basaloid (9-14%) and other mixed variants (9-10%); the sarcomatoid or spindle cell carcinoma [111, 112] ; and other exceedingly rare variants, each accounting for less than 1% of cases, such as the pseudohyperplastic, [113] the pseudoglandular, [114] the adenosquamous, [115, 116] the mucoepidermoid, [117] the clear cell [118] carcinoma, and carcinoma cuniculatum. [119] It has been remarked that accurate histological classification can provide useful prognostic information to treating clinicians. [21, 120, 121] However, identification of more unusual variants on biopsy samples is often challenging, and they are usually identified only in penectomy specimens. [105]
The histologic SCC type seems to be correlated to its biologic behavior. Human papillomavirus (HPV) DNA positivity is only weakly associated (11%) with typically keratinizing SCC and is strongly correlated histopathologically with basaloid changes (75%). [44, 122, 123] Warty [21] and clear cell [101] carcinomas are also consistently associated with HPV infection. Moreover, well-differentiated, low-grade keratinizing tumors, including the papillary and pseudohyperplastic variants, occur in older patients and are usually associated with pre-existing lichen sclerosus [113] and a low risk of metastatic spread. The types of penile SCC with a favorable prognosis, which are locally destructive but metastasize rarely, also include other verruciform variants, such as warty, verrucous, and carcinoma cuniculatum. [101] Conversely, high-risk variants with aggressive growth, high metastatic potential, and poor prognosis are the basaloid, sarcomatoid, adenosquamous, pseudoglandular, mucoepidermoid, clear cell, and poorly differentiated SCC types. A group holding an intermediate position with regard to depth of invasion, metastatic potential, and prognosis includes SCC of the usual type, [107, 124, 125] whose prognosis depends on the differentiation grade, the pleomorphic form of warty carcinomas (mixed warty/basaloid), whose metastatic potential is higher than in warty and lower than in basaloid SCCs, and the mixed forms, whose prognosis may be variable according to that of coexisting subtypes. [101]
In verrucous carcinoma, morphologically warty or verrucous, the relatively bland histologic features are often more suggestive of verruca vulgaris or pseudoepitheliomatous hyperplasia than of SCC to those unfamiliar with the diagnosis. [5, 6] Histologic examination shows massive hyperplasia of the epidermis, with marked hyperkeratosis and parakeratosis. The granular layer is prominent, with vacuolated cells that resemble the koilocytes of condyloma acuminatum. The individual keratinocytes have a large amount of cytoplasm and a large nucleus with prominent nucleoli. Atypical mitotic figures, individual cell necrosis, dyskeratosis, and multinucleated keratinocytes are rare. Intracytoplasmic glycogen is scant. Tumor projections appear as blunt masses that extend into the dermis and the deep structures to form sinuses and keratin-filled cysts. Centripetal keratinization is often present, but horn pearls are not present. A marked inflammatory lymphohistiocytic infiltrate is usually observed. Tumor cells are not found in blood vessels or lymphatics; this finding is presumably correlated with the lack of metastases. [2, 5, 6]
Histologic grading of prostatic SCC
A 3-tiered histologic grading scheme—with well, moderately, or poorly differentiated categories— should be used for SCC of the prostate.
The Gleason grading scheme can be used for the glandular component, but not the squamous component, of adenosquamous carcinomas. The glandular component should not be given a Gleason grade if histomorphologic evidence of radiotherapy or hormonal treatment effect exists. The adenocarcinoma element is often high grade (Gleason score >6), whereas the grade of the squamous portion is variable. In most cases, the squamous component is moderately differentiated, with moderate cytologic atypia. [12]
Staging of Urogenital SCC
Assessment of histologic grade and infiltration or invasion of the adjacent deep or lateral tissues aids in planning treatment. However, clinical staging is not always reliable, because tumors apparently limited to the glans and the prepuce can be under staged upon histologic examination. Moreover, a comparison of incisional biopsy and penectomy specimens from the same patients with penile squamous cell carcinoma (SCC) showed that the former may be insufficient for correctly assessing the histologic grade and deepest point of tumor invasion. [105]
Therefore, additional information obtained with complementary imaging techniques should be evaluated for accurate staging of neoplastic disease.
Clinical assessment of inguinal node metastases is also often difficult. When inguinal lymph node enlargement persists after a course of oral antibiotics, thorough imaging and histologic examinations are suggested.
The staging systems currently used for penile SCC are the Jackson anatomic classification and the tumor-node-metastasis (TNM) system (Union Internationale Contre le Cancer). [94, 126] The current TNM staging system is the American Joint Committee on Cancer 8th Edition TNM classification. [87]
TNM staging of penile SCC
SCC of the penis uses the TNM system for staging.
Tumor (T) stage is defined as follows:
-
T0 - No evidence of primary tumor
-
Tis - Carcinoma in situ (Bowen disease, erythroplasia of Queyrat)
-
Ta - Noninvasive localized SCC
-
T1 - Invasion of subepithelial connective tissue (varies by location)
-
T2 - Invasion of corpus spongiosum with or without urethral invasion
-
T3 - Tumor invading the corpora cavernosum with or without urethral invasion
-
T4 - Tumor invading other adjacent structures
Node (N) is defined as follows:
-
NX - Cannot be assessed
-
N0 - No evidence of regional node involvement
-
N1 - ≤2 unilateral inguinal nodal metastases, no extranodal extension
-
N2 - ≥3 unilateral or bilateral inguinal nodal metastases
-
N3 - Extranodal extension of any lymph node metastasis
Metastasis (M) is defined as follows:
-
MX - Cannot be assessed
-
M0 - No evidence of distant metastasis
-
M1 - Distant metastasis present
Jackson anatomic staging of penile SCC
See the list below:
-
Stage 0 - (Tis/Ta N0 M0)
-
Stage I - Tumor confined to the glans and/or the prepuce
-
Stage II - Invasion into the shaft or the corpora; no nodal or distant metastases
-
Stage III - Tumor confined to the penis; operable metastases of the inguinal nodes
-
Stage IV - Tumor involves adjacent structures; inoperable inguinal nodes and/or distant metastasis or metastases (T4 AnyN M0 or AnyT N3 M0 or AnyT Any N M1)
Staging of prostatic SCC
A staging scheme for SCC of the prostate has not been devised.
Treatment and Management
The treatment of penile squamous cell carcinoma (SCC) varies according to the clinical stage. Treatment includes radiation therapy, medical therapy (local and systemic), and surgery, alone or in combination. Currently, multimodality combined treatment is recommended. Laser therapy may also be used.
Because of the generally limited experience with SCC of the penis, considerable controversy exists as to the best form of treatment, specifically treatment for regional lymph nodes. [4, 11]
Radiation therapy
Radiation therapy with external beams, interstitial brachytherapy, or surface mold plesiotherapy (cesium [Cs] 137, iridium [Ir] 192, rhenium [Re] 188) [127, 128, 129] may be an option to treat small (T1-T2, < 3 cm), superficial, exophytic lesions of the glans or urethral sulcus in young individuals, allowing for the preservation of sexual function with a high cure rate. In case of relapse, salvage surgery may be required. [130, 131] Circumcision before therapy is recommended to minimize morbidity from the procedure.
The advantage of radiation therapy over excision has not been definitively demonstrated, [132, 133, 134] and major complications, such as urethral stenosis, fistulas, soft tissue ulceration, and penile necrosis, may occur. Some of these complications may be successfully managed with hyperbaric oxygen treatment. [135, 136]
Radiation therapy may also be used to treat advanced SCCs of lymph node metastases in patients with unresectable or recurrent lymph node disease or in patients in whom a surgical approach is not feasible. Radiotherapy used in combination with weekly Cis-platinum (chemoradiotherapy), allowing lower radiation doses, has also been suggested for unresectable locoregionally advanced disease. [137]
Adjuvant inguinal radiation therapy may be performed after surgical resection of metastatic lymph nodes, particularly in patients with multiple-node involvement or extracapsular nodal spread, [138, 137] whereas prophylactic groin irradiation should be avoided; in irradiated groins, detecting metastases is clinically more difficult, and a high rate of complications occurs after lymphadenectomy.
Radiation therapy is not indicated in the treatment of verrucous carcinoma because it may result in anaplastic changes and metastases. [139]
Local and systemic medical therapy
Early premalignant and in situ changes can be treated with topical chemotherapy (5-fluorouracil) [140] or imiquimod, [4, 141, 142] with a complete response rate of up to 57%. It requires strict follow up and must not be repeated. [101]
Systemic chemotherapy may be used according to the stage of the disease. There are 3 distinct modalities: (1) palliative chemotherapy, (2) adjuvant combined chemotherapy, and (3) neoadjuvant combined chemotherapy. [2, 3]
Palliative chemotherapy is suggested for local recurrences and for metastases when other treatments fail. It is usually delivered systemically by means of intramuscular or intravenous injection (depending on the agent used), and has been performed as monochemotherapy with bleomycin, methotrexate, or cisplatin alone or combined with other drugs for a synergistic effect.
Drugs used in combination include vincristine, bleomycin, and methotrexate (VBM therapy); cisplatin and fluorouracil (PF therapy); and cisplatin, bleomycin, and methotrexate (CBM therapy). Combinations of cisplatin and fluorouracil with taxanes (paclitaxel or docetaxel) (TPF therapy) and of cisplatin and paclitaxel with ifosfamide (TIP) have also been used, [143, 144, 145, 146, 147, 148] whereas the efficacy of a cisplatin and gemcitabine combination is disappointing. [149] Huang et al note that penile SCC is often resistant to cisplatin therapy due to a Pten deficiency in the setting of Smad4/Apc co-deletion. [150]
Adjuvant combined chemotherapy (VBM, CBM, TPF or TIP therapy) may be used to reduce the incidence of metastases in patients with node-positive disease after surgical resection. [151, 152, 153] Generally, better results are obtained with combination and cisplatin-based chemotherapy. [84, 154, 152, 155]
The finding of overexpression of epidermal growth factor receptor (EGFR) in the majority of penile SCCs, especially if HPV negative, [156] has suggested a role of anti-EGFR agents. [157, 158, 159] These novel immunotherapeutic agents have been shown to increase the chance of successful treatment. [155] Encouraging results have been obtained with cetuximab used in combination with a docetaxel [160] or paclitaxel plus cisplatin, [161] and with panitumumab. [162] The use of the vascular endothelial growth factor (VEGF)–tyrosine kinase inhibitors sorafenib or sunitinib has also been explored as a potential therapeutic option. [163]
Neoadjuvant combined chemotherapy may be attempted for locally invasive tumors (stages T3-T4) or for fixed regional node enlargement (stage N3) to reduce the neoplastic mass before surgical excision (down-staging). Better results are obtained with TIP therapy, which has been shown to offer the highest response rates. [152] Surgery is recommended only for patients responding to chemotherapy. This approach may allow for a more conservative surgical procedure than what would otherwise be needed and increase long-term survival. [148, 152, 164, 165, 166]
Other medical treatments that have been attempted or that are currently under evaluation include the following:
-
Systemic or intralesional interferon-alfa either alone or combined with surgical shaving (for relapsing verrucous carcinoma)
-
Regional intra-arterial chemotherapy with methotrexate and mitomycin C, or with gemcitabine and cisplatin [169]
-
Chemoradiotherapy with bleomycin or weekly cisplatin
-
Active immunotherapy (for HPV-positive SCCs) with tumor-infiltrating lymphocytes, HPV16/18 E6/E7–specific T lymphocytes, or immune checkpoint inhibitors (pembrolizumab) [155]
Surgical procedures
Surgical procedures for treatment of penile SCC include the following:
-
Local excision
-
Circumcision
-
Glansectomy
-
Partial penectomy
-
Total penectomy
-
Demasculinization
The last 3 procedures may be performed in conjunction with lymph node dissection. [4, 172, 173, 174]
Small (< 2 cm) and superficial lesions on the glans or the shaft (Ta, T1a) may be treated with wide wedge resection. [175, 176] Obtaining multiple deep and lateral biopsy samples for intraoperative assessment of surgical margins by frozen section is strongly recommended. [101] With improved histopathology techniques, margins of 0.5 cm may be sufficient. [101, 177] Glans skinning and resurfacing may represent an alternative option in selected cases. [178, 179, 180, 181]
Local excision with Mohs micrographic surgery has been performed with good results for small and superficial invasive carcinomas located on the glans or near the preputial sulcus. It has the advantage of preserving large amounts of healthy tissue and normal functions. Careful follow up is essential because recurrence rates are high in some series. [182]
The use of cryosurgery, either alone or combined with 5-fluorouracil, has been suggested for verrucous carcinoma. [183]
Small (< 1.5 cm) carcinomas of the distal foreskin without deep infiltration can be treated with wide circumcision. A negative surgical margin of 0.5 cm may be adequate with improved histopathology techniques. [101] Without adequate margins, the incidence of local recurrence is high (40%). However, local recurrence has no impact on long-term survival. [184, 185]
Glansectomy may be performed in verrucous carcinoma limited to the glans, [186, 187, 188, 177] whereas partial penectomy is the procedure of choice for tumors (SCC or verrucous carcinoma) at a low stage (stage I, T1-T2) that involves most of the glans or the distal third of the penis. [126, 189] Total penectomy is necessary when the lesion is in the proximal portion of the penis or when the tumor is at an advanced stage (stages II-III, T3-T4). [126]
Reconstruction techniques may help in preserving acceptable function and appearance of the penis after glansectomy or penectomy. [178, 190, 191, 192, 193] Conservative organ-sparing surgical techniques for penile SCC may reduce the emotional and functional impact of surgical treatments on patients without compromising oncological cure. [194, 195, 196]
Penectomy plus scrotectomy and bilateral orchiectomy with wide en bloc excision of the involved abdominal wall and bilateral lymphadenectomy may be required for infiltrating masses at the base of shaft. [126] However, the rarity of microscopic testicular infiltration underscores the importance of bilateral orchiectomy, even in the presence of clinically apparent scrotal invasion. [197] In such patients with advanced penile cancer and metastatic disease, an aggressive palliative resection combined with vertical rectus abdominis flap reconstruction may improve the quality of life. [198]
Lymph node dissection
Because of the significant morbidity of inguinal and pelvic node dissection [199, 200, 201] and because of the high incidence of reactive nodes, it has been suggested to perform surgical dissection of the lymph nodes only if they are enlarged and do not regress after adequate antibiotic therapy. [173] However, the current trend is to excise the primary tumor and enlarged lymph nodes at the same time, without the need of prophylactic antibiotic treatment. [101] Lymph node dissection is useful for accurate staging and improves survival. [153]
In general, if positive nodes are found during dissection of the superficial lymph nodes, bilateral dissection of the deep nodes is suggested. Bilateral pelvic lymphadenectomy should also be performed because approximately 30% of these nodes harbor tumor metastases when the inguinal lymph nodes are involved. However, pelvic lymph node dissection seems to be unnecessary in patients with 2 or fewer inguinal lymph nodes involved by a well differentiated tumor without extracapsular growth. [202] Involvement of more than 2 inguinal lymph nodes and 30% or greater lymph node density have been considered significant predictors of pelvic lymph node metastases. [203, 204]
As an alternative, some authors perform ipsilateral inguinal (superficial and deep) and pelvic lymphadenectomy with contralateral superficial inguinal node dissection to decrease the incidence of complications due to a radical approach. [131] Extensive surgical dissection is not justified if the para-aortic lymph nodes are involved (stage N4) because improved survival is not likely. [205] Surgical techniques have been proposed to reduce the morbidity of inguinal node dissection (eg, the use of a spermatic cord patch and saphenous vein sparing). [206, 207, 208]
The timing and extent of lymph node dissection are controversial because histologic investigations show that 10-20% of clinically normal nodes contain unsuspected metastases. The 5-year survival rate is as high as 84-88% in patients undergoing prophylactic inguinal lymphadenectomy versus 35-42% in those undergoing therapeutic lymph node dissection. [209, 210] A disease-specific 3-year survival rate of 84% was reported in patients undergoing early lymph node resection versus 35% in those with positive lymph nodes detected during surveillance. [210]
Histopathologic variables considered to be strong predictors of inguinal metastases are vascular, lymphatic, and perineural invasion, as well as the grade and stage of the primary tumor. [211, 212, 131, 213, 214, 215, 216] Other characteristics of the primary tumor that have been found to correlate with a higher risk of inguinal metastases include the histologic type, [217] an infiltrating growth pattern of invasion, [218, 219] and the presence of necrosis. [217]
Several novel minimally invasive diagnostic techniques have been suggested to improve detection of micrometastases. [102] In addition, several biomarkers have been investigated, although none has univocally been proven to reliably predict outcome. [220, 221] These include p53 immunoreactivity, [222] strong Ki67 labeling index, [223, 224] low E-cadherin expression, [225] high periostin [226] and annexins [227] expression, CD44 [228] and D240 immunoreactivity, [229] PmTOR and pelF4E overexpression, [230] , MYC copy number gains, [231] SCCAg, [232, 233] C-reactive protein, [234] and proliferating cell nuclear antigen (PCNA) expression. [224]
Watchful waiting may be attempted in patients with low-grade disease who are not obese and in whom an accurate clinical assessment is feasible, and who are compliant and reliable for follow-up. Patients should also have impalpable lymph nodes and superficially invasive (< 2 cm) tumors. Such patients may also be candidates for videoendoscopic inguinal lymphadenectomy, a technique that may decrease postoperative morbidity without compromising oncological control. [235, 236, 237, 238, 239, 240]
Patients with poorly differentiated and deep, invasive tumors (stage T2 or higher) are considered at high risk, especially if the tumor is proximal on the shaft and shows vascular invasion on histology; these patients are more likely than others to benefit from prophylactic dissection of the lymph nodes. [84]
Dynamic mapping of lymph nodes with technetium Tc 99m as a tracer plus blue dye followed by targeted biopsy has been introduced to restrict surgical dissection to sentinel nodes. [241] The aim is to avoid the complications of unnecessary elective regional groin dissection, which can be reserved for patients with positive sentinel nodes. [95, 96, 172, 242, 243, 244, 245] The Role of Sentinel Node Biopsy in Skin Cancer provides details on sentinel node biopsy. [246, 247] The preoperative or intraoperative use of this technique is now recommended in patients with an intermediate or high degree (≥T1G1) of differentiation cancer and nonpalpable lymph nodes. [103]
Overall, the reliability of identifying sentinel nodes has been hotly debated. [248, 249] The development of groin metastases after a negative result on sentinel node biopsy has been observed. Moreover, micrometastases may bypass the superficial nodes of the groin and drain directly into the deep groin or the iliac nodes, although this event is considered very unlikely. Findings from 2007 suggest that the isolated gamma probe technique for sentinel node penile SCC has a very low sensitivity and a high false-negative rate. Therefore, its use in combination with other techniques, such as preoperative ultrasonography with fine-needle aspiration cytology, groin exploration with intraoperative wound palpation, serial sectioning with immunohistochemical staining, visualization enhancement with fluorescent tracers (indocyanine green), and preoperative SPECT/CT, have been suggested as adjunctive tools to increase the reliability of sentinel-node identification. [250, 251, 164, 205, 252, 253, 254, 255, 102, 103, 256, 257]
In the evaluation of patients with palpable groin adenopathy, standard sentinel node biopsy is considered of low value for the detection of lymphatic spread, [232] owing to frequent false-negative results attributable to rerouting of tracer. In such cases, dynamic lymphotrophic nanoparticle-enhanced magnetic resonance imaging (MRI) with iron oxide [258] or dynamic sentinel node biopsy combined with ultrasound-guided removal of suspicious lymph nodes [259] have been suggested to improve diagnostic reliability.
Laser therapy
Destructive therapy can be administered with a carbon dioxide or a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser, alone or in combination or with an argon and potassium-titanyl-phosphate (KTP) laser, although this last option is least common. [260, 261, 262]
Laser therapy has the advantage of providing a bloodless field, [263] but it does not allow for histopathologic sampling and local recurrences can be frequent (>50%), especially with the carbon dioxide laser, which has a much shorter penetration depth.
Better results are obtained with the Nd:YAG laser, which penetrates deeper and allows coagulation of larger vessels. For this reason, accurate preoperative staging of the tumor by means of multiple deep biopsies is recommended to establish the adequacy of the depth of laser destruction. [84] This treatment should be reserved for Tis and T1 stage tumors only. [264, 168]
Further outpatient care
If patients do not undergo prophylactic dissection of lymph nodes, careful follow-up of the inguinal regions is required for 3 years. In view of the natural history of this disease, examining patients monthly during the first year of follow-up and then bimonthly during the subsequent 2 years, with quarterly pelvic and abdominal CT scans, is strongly recommended. [265]
A different schedule for physical examination has also been suggested: monthly for the first year, every 6 months for the second year, yearly for the third and fourth years, and every 5 years. [266]
More recently, and considering that recurrences mostly occur within 2 years of primary tumor, it has been recommended to perform a strict follow-up with CT or MRI imaging every 3 months in the first 2 years. [101]
Patients with an early diagnosis of a tumor at a favorable stage and with prognostic features may probably be followed less strictly than those described above, unless conservative procedures are used for treatment.
Risk-based surveillance schedules tailored to the characteristics of the neoplastic disease (stage, grade, vascular invasion, inguinal metastases), the patient (obesity, previous inguinal surgery), and the treatment protocol (penectomy vs surgical or nonsurgical phallus-sparing options, regional lymphadenectomy vs watchful waiting) have been suggested. [131] However, the timing and methods of choice for periodical post-therapeutic examinations are still controversial, and no uniform guidelines are available. [4]
Deterrence/prevention
Circumcision in infancy and a good standard of sexual hygiene are recognized as good prophylactic measures. Future directions in the prevention of penile SCC (as for cervical and vulvar carcinoma) should include further elucidation of the role of preexisting conditions, including HPV infections and genital lichen sclerosis, in the pathogenesis of the disease. Vaccination may represent a valuable preventive measure against HPV-related genitourinary cancer. [27, 267]
Prognosis of Urogenital SCC
Penile SCC
Penile squamous cell carcinoma (SCC) is the cause of fewer than 1-2% of all deaths from cancer in men in the United States. [71] Statistically significant decreased survival has been found in African American men, who tend to present with a high stage of disease. [268]
In the absence of inguinal metastases, patients with invasive SCC of the penis involving the glans or the distal part of the shaft who undergo adequate partial amputation have a long-term survival rate of 70-80%. Of patients with involved lymph nodes, 40-50% can be cured with lymph node dissection, whereas untreated patients usually die within 2-3 years. [126]
Old age, increasing primary tumor stage and grade, and lymph nodal status are associated with a lower survival rate. [269] The clinical stage of the inguinal lymph nodes is the most powerful predictor of prognosis. [270, 271, 272, 273, 274] In a study of 118 patients with penile SCC, survival appeared to be strictly related to lymph node status. [275] The 5-year survival rate was 93% for stage I, T1-3, N0, M0; 55% for stage II, T1-3, N1-2, M0; and 30% for stage III, T4 or N3 or M1. [276]
In patients with pelvic lymph node metastases, the 3-year survival rate approximates 12%. [203] Distant metastases occur in advanced disease and are predominantly found in liver, lungs, and heart. [277]
The risk increases according to the number and size (>2 cm) of enlarged lymph nodes or if fixation, extranodal extension, or ulceration is present. Another factor adversely influencing survival is bilateral involvement. [278, 279]
The thickness, depth of invasion, degree of cell differentiation of the primary tumor, [280, 270, 271, 281] and presence of vascular and perineural invasions have been identified as significant predictors of cancer progression. [125, 282, 272, 283]
Furthermore, it has been observed that invasion of cavernous bodies is a factor significantly affecting survival. [284] Studies have shown that overexpression of p53 in tumoral tissue is associated with an increased risk of tumor progression and mortality. [285, 286] A high preoperative serum circulating C-reactive protein (PCR) has also been found to be associated with poor survival. [287] Alterations in DNA sequence copy number, as evaluated by means of comparative genomic hybridization, may be correlated with the clinical outcome as well. [288]
Genetic factors may have a role; however, the findings deserve further evaluation to be considered for staging and therapeutic planning for penile SCC.
Human papillomavirus (HPV) infection does not seem to negatively influence the prognosis of patients with invasive SCC. [124, 202, 289, 290, 291]
Prostatic SCC
The mean survival for patients with prostatic SCC is not long, at 6 to 24 months. Rare patients have survived 5 years or more after chemotherapy [292] or radiotherapy. [293] There is no prognostic significance for the degree of squamous atypia (grade), previous history of adenocarcinoma, and adenosquamous versus pure SCC histomorphology. [12]
Patient Education
Early diagnosis is essential to allow for conservative treatment; therefore, patients should be advised to promptly seek medical attention in case of the onset of any penile lesion, especially if they have pre-existing conditions that increase the risk of genital cancer (eg, penile lichen sclerosus and the other disorders mentioned earlier). In such events, periodic follow-up care should be recommended for the early detection of any malignant changes.
For patient education information, see the Men’s Health Center and Cancer and Tumors Center, as well as Cancer: What You Need to Know, Skin Cancer, and Skin Biopsy.
-
Erythroplasia of Queyrat.
-
Bowenoid papulosis. Lesions at the base of the shaft.
-
Condylomata acuminata.
-
Squamous cell carcinoma arising on genital lichen sclerosus.
-
Pseudoepitheliomatous, keratotic, and micaceous balanitis.
-
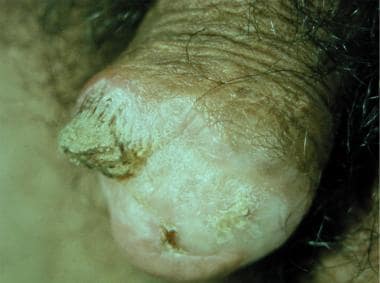
Penile horn.
-
Papillary squamous cell carcinoma on the penis.
-
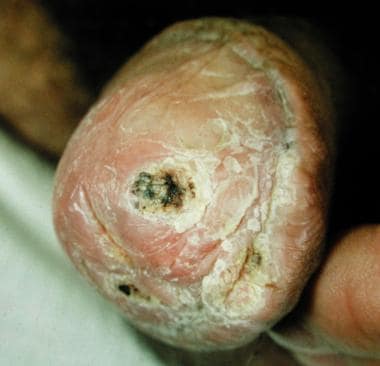
Ulcerated squamous cell carcinoma on the glans.
-
Verrucous carcinoma.
-
Verrucous carcinoma.
-
Verrucous carcinoma.
-
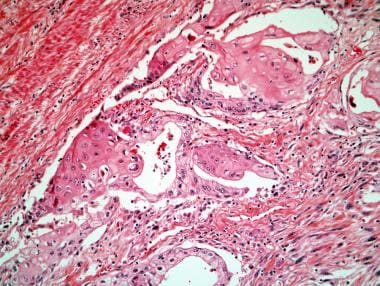
Squamous cell carcinoma of the prostate.
-
Squamous cell carcinoma of the prostate, with keratinization
-
Adenosquamous carcinoma of the prostate
Tables
What would you like to print?
- Practice Essentials
- Pathophysiology of Penile SCC
- Pathophysiology of Prostatic SCC
- Epidemiology
- Diagnosis of Urogenital SCC
- Workup for Urogenital SCC
- Histologic Findings in Urogenital SCC
- Staging of Urogenital SCC
- Treatment and Management
- Prognosis of Urogenital SCC
- Patient Education
- Show All
- Media Gallery
- References