Overview
Pathogenic filarial parasites affect the lives of millions of people, especially those living in tropical countries, and may cause significant dermatologic manifestations. The filarial parasites that pose the most serious public health threats are Wuchereria bancrofti,Brugia malayi, Brugia timori, Onchocerca volvulus, and Loa loa. All of these cause cutaneous manifestations. Filarial worms of the genus Dirofilaria and zoonotic Onchocerca species, normally parasitic in animals, occasionally enter a human host and undergo partial or aberrant development. These may cause cutaneous or subcutaneous manifestations. Another filarial nematode, Mansonella genus, is also a public health threat.
Related Medscape articles include Filariasis and Dirofilariasis.
Onchocerciasis
Onchocerciasis is caused by infection from the nematode Onchocerca volvulus transmitted by the Simulium black fly.
Onchocerciasis is most prevalent in sub-Saharan Africa but is also endemic in parts of the Arabian Peninsula and parts of Latin America. In highly endemic areas, O volvulus infection is acquired early in life, and over 90% of inhabitants may be infected by age 20 years.
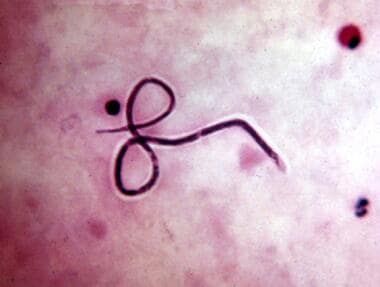
Third-stage larvae (L3) enter human skin from a fly's proboscis (see the image below).
Pathogenesis
The lifecycle begins when a black fly ingests larva or microfilariae of O volvulus from the skin of an infected human host. Within the fly, the microfilariae pass through two molts to the third infective stage (L3) over a period of 1-3 weeks. At the next blood meal, the fly deposits the larvae within the skin of a new human host. [1] The larvae remain in the dermis and subcutaneous tissue where they undergo two additional molts to mature into hairlike adult worms.
After maturation, the female adults, now 30-80 cm in length, become encapsulated in deep subcutaneous nodules or onchocercomata (onchocercomas) in the dermis and deep fascial planes. Male adults migrate between the nodules, where they fertilize sedentary females. Females then release thousands of microfilariae, which move into the surrounding tissues or eyes (causing blindness) where they can be ingested by feeding black flies to complete the life cycle (see the image below). Most microfilariae live in the subepidermal layer of the skin, where they can be taken up by the feeding blackfly. Each fertile female can produce millions of microfilariae during her lifetime, and the worms can live for 10-15 years.
Most clinical manifestations associated with O volvulus infection are related to the chronic effects of repeated episodes of inflammation. Microfilariae are most abundant in the skin but also invade the eyes, lymph nodes, and other deep organs, where they may produce severe and progressive inflammatory lesions. Inflammatory reactions follow the death of microfilariae and are caused by the secretion of toxic products by granulocytes, the deposition of immune complexes in the tissues, and by inflammatory mechanisms induced by release of Wolbachia (bacteria infecting the filariae)‒derived products.
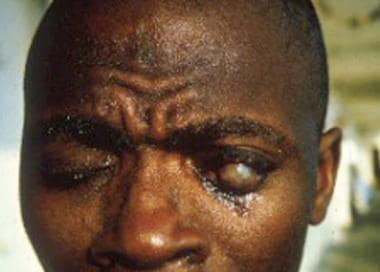
Wolbachia is an endosymbiont bacteria found in many filariae that assists in reproduction and survival, including in O volvulus. Dead microfilariae in the eyes release Wolbachia that cause inflammation and can lead to interstitial keratitis, band keratopathy, faint iritis or iridocyclitis, posterior synechiae, distortion of the pupil, chorioretinal scarring of the fundus (Hissette-Ridley fundus), and other eye damage—leading to the expression river blindness. [2, 3]
Clinical presentation
The blackfly bite may leave a bleeding point on the skin, with surrounding erythema and severe itching. Multiple bites in a nonimmune person produce pruritus, pain, and urticaria.
Patients with different onchocercal skin pathology have different O volvulus–specific antibody isotypic responses. Evidence also indicates that differences seen in the severe and mild strains of O volvulus relate to the amount of Wolbachia symbiote found in the different strains. [4] HIV infection appears to increase the severity of onchocercal dermatitis. [1, 5]
The earliest and most prevalent skin symptom of onchocerciasis is pruritus, and, thus, secondary changes such as excoriation, superficial ulceration, and secondary infection can occur (see the images below). Pruritus is most severe over the lower trunk, pelvis, buttocks, and thighs. Classically, it is confined to one anatomic quarter of the body.
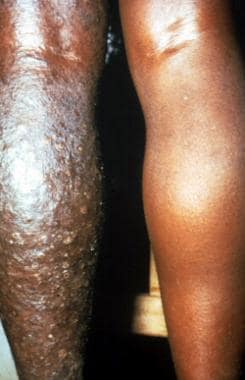
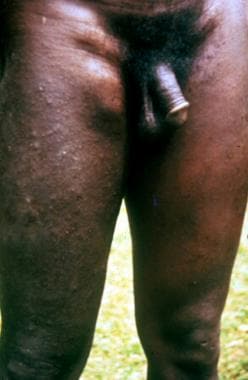 One anatomic quarter of body (leg) markedly affected by edema and papule formation of onchodermatitis.
One anatomic quarter of body (leg) markedly affected by edema and papule formation of onchodermatitis.
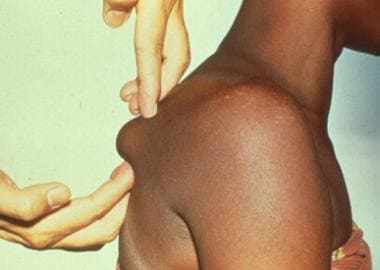
The most distinctive cutaneous finding is a firm, mobile, subcutaneous nodule termed an onchocercoma. They are typically located over bony prominences and the head—they are usually 1-3 cm in diameter (see the image below).
They are most common over bony prominences (ie, skull, scapulae, ribs, elbows, trochanters, iliac crests, sacrum, knees) and in deeper sites near joints and bones or between muscles (see the image below). [6]
Usually, nodules are close to each other and form large masses with small satellite nodules. This gives a lobulated feel to the tumor. Removal may reveal more nodules than those initially palpated. With experience, O volvulus nodules can be distinguished from lipomata, cysts, lymph nodes, juxta-articular nodes in late yaws, and other nodules.
Aside from the onchocercoma, the five classic skin eruptions associated with onchocerciasis are as follows:
-
Acute papular onchodermatitis
-
Chronic papular onchodermatitis
-
Lichenified onchodermatitis
-
Skin atrophy
-
Skin depigmentation.
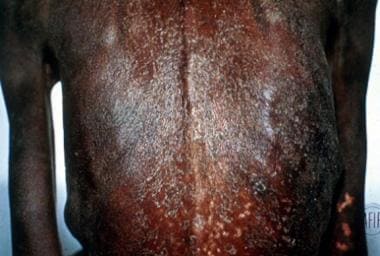
Acute papular onchodermatitis consists of pruritic papules 1-3 mm in diameter that may progress into vesicles or pustules. Chronic papular onchodermatitis consists of flat-topped, hyperpigmented, pruritic papules typically 3-9 mm in diameter. Lichenified onchodermatitis is usually confined to one limb and consists of hyperpigmented lichenified plaques. Skin atrophy leaves the skin aged, wrinkled, and often with less hair and hypohidrotic. [1] The depigmentation of onchodermatitis is peculiar. Often referred to as “leopard skin,” there are patches of complete pigment loss except for perifollicular islands of retained normal pigmentation—similar to the perifollicular islands of normal pigment in patients with scleroderma depigmentation (see the image below).
This can be mistaken for leprosy, but unlike lepromatous lesions, no sensory changes are present. The leopard skin can also mimics forms of pinta, malnutrition, and vitiligo, any of which can exist concomitantly. Finding nodules can aid in these distinctions.
Over time, the lymphatics may become irreversibly damaged. Lymphedema may occur, leading to elephantiasislike changes and, in the groin, massive edema and “hanging groin” changes similar to lymphatic filariasis (see Lymphatic Filariasis below).
In Latin America, a variety of more acute skin lesions occurs, while chronic skin lesions are less common. Acute lesions, a rare occurrence in Africa, include the erisípela de la costa, a macular rash with edema of the face (see the image below), and mal morado, a red-brown discoloration seen mainly on the trunk and upper limbs.
In the Arabian Peninsula, and less commonly in Sudan, West Africa, Guatemala, and Ecuador, some patients develop a hyperpigmented skin change termed sowda. Sowda is a form of hyperreactive onchocerciasis usually limited to one limb, but it can affect more than one limb or even the trunk. The involved skin is itchy, swollen, darkened, and covered with scaling papules, and regional lymphadenopathy is present (see the image below). [7]
Diagnosis
The standard of diagnosis is microscopic examination of bloodless skin snips for microfilariae. A skin snip is a specialized way of obtaining a biopsy specimen. After an area of skin is wiped with alcohol, it is elevated with the tip of a needle and a small segment of the tented skin is shaved off with a razor blade or scalpel. This piece of skin is placed on a slide under a cover slip and immersed in isotonic saline. After 15-20 minutes, the preparation is examined for the presence of motile microfilariae that emerge from the skin tissue. The diagnosis may also be made through the microscopic examination of subcutaneous nodules.
Individuals with no evidence of O volvulus microfilariae in the skin or eyes can be challenged using the Mazzotti test. The patient is given a single oral dose of 25-50 mg of diethylcarbamazine (DEC). Patients should have a complete ocular examination for microfilariae before considering the performance of this test because inflammation after DEC administration may cause irreversible damage to the posterior segment of the eye. Infected individuals develop intense pruritus between 30 minutes and 24 hours after the test, which can then be accompanied by erythema, edema, and papular dermatitis. The presence of M streptocerca may cause false-positive reactions.
Assays to detect specific antibodies to Onchocerca and polymerase chain reaction to detect onchocercal DNA in skin snips are now in use in specialized laboratories and are highly sensitive and specific. Although current serologic assays have limited ability to discriminate past exposures from current infections, the detection of O volvulus DNA in microscopically negative skin snips is useful in individuals with subtle infections.
Treatment of onchocerciasis
Medical therapy
Ivermectin is the drug of choice for the treatment of onchocerciasis. It functions as a single dose and is a rapidly effective microfilaricide for O volvulus. Unlike DEC, ivermectin does not produce a significant Mazzotti reaction in onchocerciasis, most likely because it acts by paralyzing the microfilariae in the skin tissue spaces and lymphatics. [8] They are then swept away into the local lymph nodes, which may swell up, and only cause some local limb edema. On the other hand, DEC unmasks the microfilariae in the tissue spaces and the exposed Wolbachia organisms within them, leading to the significant immune inflammatory response.
Ivermectin kills microfilariae within female worms, as well as those in human tissues. Dead microfilariae within the uteri of the female worm degenerate and prevent further microfilarial production for 6-12 months. Ivermectin not only kills microfilariae, it is also a microfilarial suppressant. Data suggest that ivermectin may reduce the development of infective larvae to young adults. [9] Two to three months after ivermectin therapy, microfilariae usually disappear from the eyes, halting the advancement of ocular lesions. Patients with visual-field defects, anterior segment disease, and optic nerve damage benefit. Advanced disease of the posterior segment (eg, chorioretinitis) does not improve but stabilizes with treatment. [10]
The optimal dose of ivermectin is 150 mcg/kg (usually a 6-mg tablet twice for adults) given once orally. The dosage can be repeated every 6-12 months (the 6-month regimen has a higher antiparasite effect and causes fewer adverse reactions after the second and third treatments. Multiple dosing at intervals of 3 months may have a slow but steady attrition effect on the adult worms. [11]
Use of ivermectin in children younger than 5 years, in pregnant or breastfeeding women (after a 1-week limit), and in patients with epilepsy is now permitted. However, the drug is prescribed for patients with other severe illnesses and should not be given during outbreaks of meningitis. [12]
Although up to 30% of patients experience adverse reactions after the first dose, ivermectin seems safe for large-scale use and is proving to be an effective method for reducing the human microfilarial reservoir sufficiently to control transmission by the Simulium black fly. [13, 14, 15] Caution is needed in areas endemic for both loiasis and onchocerciasis because ivermectin can initiate serious adverse effects, including central nervous system damage in patients with high L loa microfilarial loads (microfilariae counts >2500/mL). [16, 17, 18]
Studies have demonstrated that after many rounds of ivermectin treatment, some patients continue to have surprisingly high microfilarial counts. This suboptimal response to ivermectin treatment may indicate a developing drug resistance. [19]
Moxidectin is an antiparasitic drug approved by the US Food and Drug Administration (FDA) in June 2018 to treat onchocerciasis in patients aged 12 years or older. The World Health Organization initiated clinical trials for use in onchocerciasis in 2009. Moxidectin is closely related to ivermectin, but it has a more sustained reduction in microfilarial levels. FDA approval was based on a double-blind, parallel group, superiority trial (n=1472) that compared moxidectin (8 mg PO once) with ivermectin (150 mcg/kg PO once). [20] The trial took place in Ghana, Liberia, and the Democratic Republic of the Congo. Results showed skin microfilarial loads (ie, parasite transmission reservoir) were lower from month 1 to month 18 after moxidectin treatment than after ivermectin treatment, with an 86% difference at month 12. Moxidectin would therefore be expected to reduce parasite transmission between treatment rounds more than ivermectin could, thus accelerating progress towards elimination.
The use of tetracyclines to kill the endosymbiotic Wolbachia bacteria appears to be effective for O volvulus and other filarial worms. [21] The addition of oral doxycycline at 100 mg/day given for 6 weeks (or 4 wk at 200 mg/day) from the start of ivermectin to kill off the Wolbachia helps to prevent treatment reactions and completely blocks the development and production of new microfilariae. [2] Since adult worms sterilized by doxycycline do not produce microfilariae, microfilariae should not reappear after these ivermectin treatments unless the patient acquires new worms by reinfection. Doxycycline treatment may be particularly beneficial for people with onchodermatitis and pruritus who would otherwise require repeated treatment with ivermectin over a period of years. In addition to its sterilizing effect, the 4-week, 200-mg/day doxycycline regimen also kills approximately 60% of adult O volvulus worms. Doxycycline has little or no effect on microfilariae that are already in the skin or eyes. [22]
DEC is a microfilaricide with no effect on the adult worm. Ivermectin has many advantages over DEC, and DEC should not be used if ivermectin is available. DEC produces Mazzotti reactions that become severe in heavily infected persons. Slow titration of the DEC dose is advisable, beginning with one 50-mg tablet on the first day and followed by two 50-mg tablets over the next 7-10 days. Oral and perhaps systemic steroid therapy is advisable to reduce the Mazzotti reaction. A low dose of dexamethasone (3 mg/day), begun after the onset of the Mazzotti reaction, modifies the progression of the reaction without interfering with the macrofilaricidal efficacy of DEC. [23] This suggests that the Mazzotti reaction is not directly caused by death of microfilariae, but rather is a response of the immune system.
Suramin is both a macrofilaricide and a microfilaricide. It is given intravenously, starting with a test dose of 100 mg of fresh 10% solution over 2 minutes. If no hypersensitivity develops, weekly dosages of 0.2 g, 0.4 g, 0.6 g, 0.8 g, and 1 g are given to adult patients. Rarely, patients experience worsening of eye lesions, exfoliative dermatitis, kidney damage, a Mazzotti-like reaction, and/or death. Thus, the use of suramin requires great caution and currently is not recommended.
Nodulectomy
A useful adjunct to chemotherapy is removal of the palpable nodules. This is popular in Guatemala, Ecuador, and Mexico. In Africa, nodulectomy has never been practiced widely because the nodules tend to be deeper and located near delicate joint spaces. Alternatively, chloroquine can be injected into young nodules to kill the worms.
Lymphatic Filariasis
Lymphatic filariasis is endemic in nearly 80 countries, with an estimated 1 billion people worldwide at risk of infection. Lymphatic filariasis is endemic in Africa, Asia, the Indian subcontinent, the western Pacific Islands, focal areas of Latin America, and the Caribbean—particularly Haiti and the Dominican Republic. The burden of disease is 120 million people already infected, with more than 40 million who are incapacitated or disfigured by the disease. [24] A testament to the burden of the disease is that in India, 3.8-8% of potential male labor capacity is estimated to be lost due to lymphatic filariasis morbidity; in Ghana, that number was estimated to be over 7%. [25] . In the Americas, endemic foci persist on the island of Hispaniola and in coastal areas of Guyana and northeastern Brazil. Infection with Brugia malayi is limited to Asia (India, Malaysia) and several western Pacific island groups (Indonesia and the Philippines). Both China and the Republic of Korea were considered endemic until recently, but have declared elimination of lymphatic filariasis as a public health problem in 2007 and 2008, respectively
Three species of filarial parasites commonly inhabit the lymphatic system of humans, W bancrofti, B malayi, and B timori. All three of these parasites can produce significant direct dermal injury. About 90% of infections are caused by W bancrofti, for which humans are the only natural host. There are fewer than 10-20 million persons who are infected with B malayi. B timori is only found on the islands of southeastern Indonesia. Very rarely, other Brugia parasites of animals (eg, Brugia pahangi) cause aberrant infections in humans. Occupation and socioeconomic status are important risk factors as filariasis affects primarily persons of the lowest socioeconomic levels. Infection occurs more frequently in young men than women, but the role of gender in susceptibility to infection and disease is poorly understood.
Pathogenesis
The major symptoms of bancroftian and Malayan filariasis relate to damaged lymphatics. Structural damage usually occurs only if hundreds of thousands of mosquito bites occur over years. Adult worms live in the lymphatic system and produce microfilariae. The worms induce local reactions by undefined mechanisms and cause dilatation and tortuosity of lymphatic vessels, hypertrophy of vessel walls, loss of valvular function, and backflow of lymph. Structural and functional abnormalities of lymphatic channels may develop even in entirely asymptomatic individuals with microfilaremia. [1, 26]
Like many filarial parasites, an essential part of the pathophysiology of W bancrofti and Brugia species is the presence of an obligate intracellular bacterial endosymbiont, Wolbachia, which is essential to reproduction, development, and survival of the worm. A clue to Wolbachia’s importance came in 1912, when Wise and Minett in British Guiana (Guyana) provided the earliest evidence of the role of bacteria in aggravating and promoting the clinical manifestations of filarial infections. In 1932, O'Connor presented evidence suggesting that living worms produced no serious pathology but that dead and dying worms can initiate an immunologic response that leads to clinical elephantiasis independent of any secondary bacterial infection. [27] Like with onchocerciasis, this realization has led to treatment advances.
Clinical presentation
Depending on the stage of disease, lymphatic filariasis has different manifestations.
The subacute infection may be clinically asymptomatic, but the infected individuals may have underlying lymphatic damage, with microscopic hematuria or proteinuria found in infected persons who are asymptomatic.
Acute presentations of infection include acute adenolymphangitis (ADL) and acute filarial lymphangitis (AFL). ADL presents as a plaquelike area of relatively diffuse cutaneous inflammation along with episodes of fever and inflamed lymph nodes in the groin and axillae. It has been compared with erysipelas and lymphangitis in its clinical presentation. These episodes often last 3-15 days each, may recur several times each year, and lead to further progression of chronic lymphedema. The etiology of ADL is unclear. Theories include an inflammatory reaction to adult filarial worms in the lymphatic system, microfilariae in the blood, and a reaction to bacterial pathogens such as Streptococcus A or Staphylococcus due to impaired lymphatic drainage. [1, 26]
AFL involves acute inflammation of a lymphatic vessel that progresses distally along the vessel and is thought to be caused by the death of the adult worm either spontaneously or as the result of medication. Patients present with small, tender nodules at the site of the dying worms. Patients also give a history of pain, erythema, and tenderness in the affected lymph node region for hours or a day prior to the onset of lymphangitis. Typically, AFL is not as severe or harmful as ADL. [1, 26]
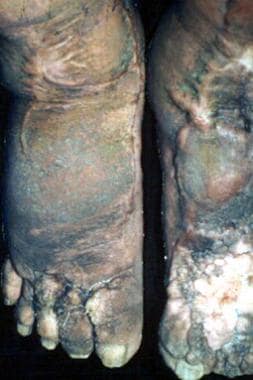
Chronically compromised lymphatic function leads to lymphedema that is initially reversible. Continued inflammation in the compromised lymphatic system by persistent parasites, and especially by complicating localized bacterial and fungal superinfection of skin, causes the lymphedema to become irreversible. As lymphatic damage progresses, the initially transient edema and anatomic distortion transform into the permanent changes of elephantiasis (see the images below). The skin becomes coarse, verrucous, and thickened. Cracks, papillomatous changes, and folds characterize the endstage (see the image below). [26] The edema, once pitting, becomes firm and permanent. These changes usually have an asymmetric distribution and often occur from the groin on downwards.
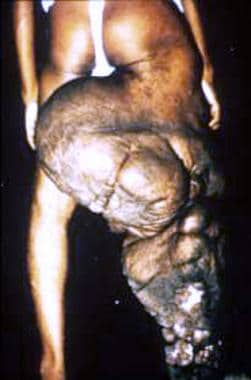 Nodular and hyperplastic changes cause massive distortion to leg of an Indian man with bancroftian filariasis.
Nodular and hyperplastic changes cause massive distortion to leg of an Indian man with bancroftian filariasis.
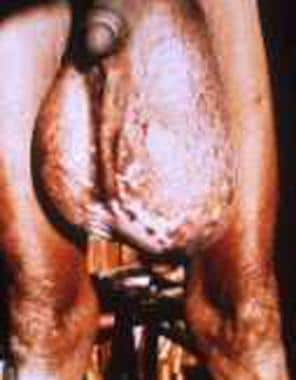
The location of lymphatic damage determines the site and type of clinical manifestations. Chronic lymphedema leads to chronic elephantiasis of limbs, breasts, and genitalia. Obstruction of the lymphatics in the groin leads to hydrocele and/or chyluria (see the image below).
Reported clinical manifestations and sequelae include abscesses, ulcers, breast nodules, pleural effusions, constrictive pericarditis, and fibrosing mediastinitis. Massive genital edema leads to the hanging groin presentation (see the image below).
Differential diagnosis
Differential diagnosis includes lymphedema from chronic medical conditions (eg, recurrent bacterial lymphangitis, malignancy, cardiac or renal failure), congenital lymphedema (Milroy disease), and, in tropical volcanic highland areas in Africa, Northern India, and Central and South America, a condition named podoconiosis.
Podoconiosis is often mistaken for lymphatic filariasis owing to its similar morphology and that the afflicted populations are also from tropical locations. Podoconiosis is a form of acquired lymphedema that results from chronic inflammation of the lymphatics from walking barefoot in soil of volcanic origin. This soil has a high concentration of metal particles that penetrate the sole and cause inflammation of the lymphatics. It is usually differentiated from filariasis clinically by the distribution—occurring symmetrically on the feet most prominently, with changes confined to below the knee and without groin involvement. [28, 29]
Diagnosis
W bancrofti infection may be diagnosed via testing for circulating antigen via enzyme-linked immunosorbent assay (ELISA) testing and a rapid-format immunochromatographic card (ICT) test. These tests are specific and sensitive, and the ICT also is portable and inexpensive. Neither test depends on periodicity of the filarial presence. There is no antigen test for brugian filariasis. [1, 26]
Microfilariae can be found in blood, hydrocele fluid, and occasionally in other fluids, such as urine. Diagnostic sampling must take into account the periodicity of microfilariae. Microfilariae of W bancrofti circulate at highest concentration at night; therefore, blood specimens should be collected between 22:00 hours and 02:00 hours. Microfilariae of the three species are distinguished morphologically. The standard for diagnosis is microscopic detection of microfilariae on a thick blood film with Giemsa or hematoxylin staining. Eosinophilia (>10%) is commonly found in patients.
Imaging studies also have a role in detection of filaria. High-frequency ultrasound in conjunction with Doppler examination of the superficial lymphatics of the extremities and female breast or the intrascrotal lymphatics in men has demonstrated adult worms within dilated lymphatics. Filaria motility in vivo has been termed the “filaria dance sign”. [30] In addition, radionuclide lymphoscintigraphic imaging of the extremities has demonstrated lymphatic dilatation and dysfunction in both asymptomatic persons and in those with lymphedema. Also, adult worms localized in lymphatic vessels or lymph nodes have been found in pathology specimens. The lethal effects of antifilarial drugs on adult worms can be directly monitored in vivo by ultrasound.
Treatment and prevention of lymphatic filariasis
Single-dose combination drug regimens have mostly replaced traditional 12-day courses of diethylcarbamazine (DEC) for the treatment of lymphatic filariasis. A single annual dose of albendazole (400 mg) with either DEC (6 mg/kg) or ivermectin (200 µg/kg) reduces microfilariae counts to very low levels for at least 24 months (in the absence of reinfection). Albendazole with DEC is believed to have better macrofilaricidal activity than albendazole with ivermectin. However, since DEC is contraindicated in patients with onchocerciasis, the albendazole/ivermectin regimen is preferred for the treatment of lymphatic filariasis in areas that are co-endemic for onchocerciasis. [1]
DEC kills microfilariae and it also kills a proportion of adult W bancrofti,B malayi, and B timori. DEC does not kill microfilariae directly, but it seems to modify them so that the host's immune system may detect and then clear them from the blood. DEC is administered orally with food. Microfilaria counts decrease quickly after DEC treatment (sometimes to undetectable levels), but counts tend to increase slowly a few months after treatment. Circulating filarial antigen (CFA) levels decrease after DEC treatment, but total clearance is unusual after a single course of treatment. This indicates that some adult worms survive the treatment.
Adverse events are common following treatment with DEC and may occur as early as a few hours after the first dose of the drug. Adverse events tend to be less severe in bancroftian filariasis than in brugian filariasis. Clinically significant adverse events are not seen in uninfected individuals, so the adverse events are believed to be related to systemic and local responses to dying filarial worms. The intensity of systemic adverse events is correlated with microfilaria counts, and the most severe reactions occur after the first treatment. Systemic adverse events include fever, headache, malaise, arthralgias and myalgias, dizziness, anorexia, and vomiting. [1, 26]
Localized adverse events (related to death of adult filarial worms) are less common than systemic adverse events. These can include lymphadenitis (sometimes with suppuration and drainage) and transient lymphedema. Male patients with bancroftian filariasis sometimes experience scrotal pain and/or epididymitis after treatment; they may develop new hydroceles that usually resolve over a period of weeks. Local adverse events tend to occur later (often ≥1 wk after treatment) and last longer than systemic adverse events. DEC can cause severe adverse events in patients with onchocerciasis and in loiasis patients with high blood microfilaria counts.
Ivermectin is a potent microfilaricide that temporarily sterilizes adult worms of W bancrofti and B malayi without killing the adult worms. Systemic adverse events after ivermectin are generally similar to those mentioned above for DEC, but localized adverse events are uncommon. Ivermectin should not be used in pregnant women or in children younger than 5 years. The major role of ivermectin in lymphatic filariasis is for treatment and control of lymphatic filariasis in areas that are co-endemic for onchocerciasis and/or loiasis. Since it has no macrofilaricidal effect, repeated annual or semiannual treatments may be required for years to suppress microfilaremia.
With albendazole, microfilaria counts also decrease slowly—over a period of months by 50% or more after a single oral dose of 400 mg. Albendazole also has activity against adult filarial worms. Treatment with high-dose albendazole (400 mg twice daily for 21 days) was effective for killing filarial worms, but this regimen caused an unacceptably high rate of local adverse events. As noted above, albendazole is usually administered in combination with either ivermectin or DEC.
More recently, doxycycline has shown efficacy via killing of the endosymbiotic bacteria Wolbachia. Doxycycline works by clearing Wolbachia bacteria from filarial species such as W bancrofti and B malayi that require the endosymbiont for reproduction and viability—killing the adult filaria. This is in contrast to its effect in onchocerciasis, in which tetracyclines prevent microfilariae development. [31] The drug has little direct effect on microfilariae, and microfilaria counts decrease slowly over a period of months after doxycycline treatment. Long courses of doxycycline treatment are required to clear Wolbachia from filarial worms, but results of this treatment are impressive. Macrofilaricidal activity of doxycycline may be enhanced by a single dose of DEC 3 months after the course of doxycycline. Although doxycycline can cause adverse events such as photosensitivity, vaginal candidiasis, and esophagitis, it has not been reported to cause systemic or localized adverse events related to dying filarial worms. Thus, doxycycline is an attractive option for treating individuals with lymphatic filariasis. The long treatment course required and the fact that doxycycline is contraindicated in pregnant women and children younger than 9 years make it unattractive for routine use in filariasis elimination programs based on mass drug administration. Ongoing research is testing whether new drugs or drug combinations can shorten the time required to clear Wolbachia from filarial worms.
There are no official recommendations for use of combination regimens to treat patients with lymphatic filariasis. However, it is reasonable to provide single-dose combination regimens at 6-month intervals for patients who live in areas with ongoing transmission until microfilaria tests are negative in order to treat possible reinfections. There is no evidence that patients with clinically evident filariasis require more treatment than asymptomatic patients with microfilaremia.
Treatment of patients with microfilaremia (and amicrofilaremic patients with positive CFA tests) may prevent the development of lymphatic damage by killing adult worms. While some patients with early disease (pitting edema, small hydroceles) may notice clinical improvement after treatment, antiparasite treatment may not improve advanced disease because of severe lymphatic damage (large hydroceles, brawny edema or elephantiasis). The death of adult worms following treatment may trigger AFL attacks. For this reason, treatment is not recommended during acute filarial fever episodes, because it may kill additional worms and further exacerbate inflammatory reactions.
The aggressive treatment of chronic lymphedema and elephantiasis can lead to marked improvement of symptoms. The most important local treatments are those measures that prevent superficial bacterial and fungal infection. Additionally, patients should use limb elevation, special massage techniques, and elastic stockings to protect the affected extremity.
Patients with severely damaged extremities may benefit remarkably from surgical decompression of the lymphatic system through endovenous shunt surgery followed by excision of redundant tissue. Surgical correction or repeated drainage is the treatment for hydroceles. Surgical correction sometimes is used to correct chyluria. Of interest, the diagnostic lymphangiography itself often appears to terminate the leak of chyle into the urine, probably because of its sclerosing effects on the lymphatic vessels that have ruptured into the renal pelvis.
Loiasis
Loiasis is caused by the filarial nematode Loa loa, transmitted from the bite of the Chrysops mango fly (see the image below). Loiasis is widely distributed in West and Central Africa, and humans are the only important reservoir. Loiasis transmission mainly takes place during the wet season. In addition to the bite of the mango fly, entry of the infective larvae increases the severity of the swelling and itching above that experienced from the bite of an uninfected fly. There does seem to be a genetic predisposition to acquisition of L Loa infection. [32]
Later, as infective larvae move away under the skin and molt, they often give rise to discrete red urticarial papules in the affected part of the skin. The larvae have a predilection for loose tissues such as the eye and periorbital region, frenulum of the tongue, and the genitalia. These filiariae may also be noticed subcutaneously with the appearance of cutaneous larva migrans classically most visible in the fingers and breasts. As these larvae mature over the next 3 months or more, the patient may experience vague symptoms such as pain or temporary swelling of a limb, itching, paresthesia, a creeping sensation, and urticaria.
Transient migratory angioedema is a common and classic manifestation of loiasis and is termed Calabar or “fugitive” swellings. The high incidence of loiasis and the subsequent angioedema in the town of Calabar in southeastern Nigeria gave rise to the name Calabar swelling.
Calabar swellings are thought to be a hypersensitivity response to antigenic material released and left behind by a migrating, developing, or adult worm. They are more common in expatriate Europeans than in native Africans. They are most common on the face and extremities (see the image below). Mild local trauma may precipitate their appearance, and swellings recur more often during hot summer months than during colder seasons. Typically, an area develops a prodrome of pain or itching, followed within hours by the development of a 10- to 20-cm area of nonpitting edema. The edema lasts from a few days to several weeks and is usually painless, except when the location of the swelling causes restriction of joint movement or nerve compression. They can recur at irregular intervals and tend to recur at the same sites. Peripheral nerve compression is most common in the region of the carpal tunnel. [1]
L loa infection also causes a characteristic localized lymphadenitis that generally is found incidentally. Other signs include diffuse edema of the hand or forearm, generalized urticaria, fever, irritability, and confusion. Loiasis also can cause orchitis, scrotitis, and inflammation of the spermatic cord. Rarely, patients develop nephropathy, cardiomyopathy, or pulmonary damage, including pulmonary infiltrates and pleural effusion. Acute arthritis with effusion is an uncommon complication, and some evidence suggests that loaiasis can aggravate psoriasis. Eosinophilia is common and may reach 50-80% (10,000/µL); however, no correlation exists between the number of microfilariae in the blood and the eosinophilic response. [1]
Diagnostic testing
Testing for loiasis microfilariae is done on a daytime, Giemsa-stained blood smear or via demonstration of an adult worm removed from subcutaneous or conjunctival tissue. The optimal time for taking a blood sample for microfilaria testing is around 12:00 hours, when the concentration of microfilariae in the peripheral blood is at its peak. Polymerase chain reaction testing for loiasis is also approved in the United States. [1] An exciting development is the advent of an innovative cell-phone–based device, "the LoaScope", used to rapidly identify people with high L Loa microfilariae counts (>20,000 mf/mL)—this has been shown to be useful in excluding these patients from potentially harmful ivermectin therapy. [33]
Treatment
Live adult worms can be extracted as they traverse the eye. In patients with few or no circulating microfilariae, diethylcarbamazine (DEC) at a dose of 8-10 mg/kg/day in three divided doses for 21 days is the drug of choice. The drug is not directly toxic to the parasites but works in conjunction with the host immune responses to kill microfilariae and adult worms. DEC is curative in most cases, although multiple courses of therapy are often necessary, and relapses have been documented as late as 8 years after treatment.
Mild adverse effects of DEC treatment include recrudescent Calabar swellings, urticaria, arthralgias, fever, and right upper quadrant tenderness. Adverse events are common during the first few days of therapy and generally respond to antihistamines or a short course of corticosteroids. DEC should not be used in patients with concomitant onchocerciasis, because of the risk of severe cutaneous and ocular reactions in these individuals. Serious complications of DEC treatment, including renal failure, meningoencephalitis, coma, and death, are most common in patients with high numbers of circulating microfilariae (usually >8000/mL but possible when >2500/mL) and are thought to be due to the massive release of antigens from dying microfilariae. Although advocated in the past, neither a gradual increase in DEC dose nor pretreatment with corticosteroids is completely effective in preventing encephalitis in such patients. Thus, for patients with high L loa microfilariae loads, pretreatment with cytapheresis or albendazole 200 mg twice daily for 21 days is recommended.
Albendazole, at a dose of 200 mg twice daily for 3 weeks, can reduce L loa microfilaremia by approximately 80% over the course of several months without adverse effects. Shorter courses of higher dose albendazole are less effective. Albendazole has been used successfully in DEC-refractory L loa infection.
Ivermectin, the treatment of choice for onchocerciasis, has activity against L loa microfilariae, but little, if any, effect on adult worms. Consequently, since ivermectin is not curative and causes similar adverse effects to those seen with DEC in patients with high microfilarial loads, it should not be used for the treatment of loiasis. Doxycycline is ineffective in loiasis owing to the absence of the bacterial endosymbiont Wolbachia.
Mansonelliasis
Mansonella perstans
Infection with M perstans is common in Africa, where it affects, humans, gorillas, and chimpanzees. It is endemic in much of tropical Africa, from Senegal, east to Uganda, and south to Zimbabwe. Biting midges are the vector for this filarial worm. The midge species Culicoides austeni and Culicoides grahamii are the major natural insect vectors. In South America, infections with M perstans develop in persons living along the Atlantic Coast, from Panama and south to Argentina, including Trinidad.
Most infected with M perstans are asymptomatic. Individuals new to an endemic area tend to experience more symptoms than local inhabitants. Cutaneous findings include subcutaneous swellings of the arms, shoulders, and face similar to Calabar swellings in loiasis, urticaria, and pruritus. Extracutaneous findings include abdominal pain, pleuritic, arthralgias, and fatigue. Degenerating worms incite inflammatory exudates composed of eosinophils, neutrophils, plasma cells, and macrophages. The end result is focal abscesses that can develop into granulomas and scars.
Some patients may develop periorbital edema and conjunctival irritation, sometimes with granulomatous nodules in the conjunctiva. Acute periorbital inflammation is common in Uganda (and in some patients in Nigeria and Sudan) and is termed “bung-eye” or “bulge-eye”.
Microfilariae of M perstans are primarily in peripheral blood (see the image below); they are infrequently found in cerebrospinal fluid and urine. Peripheral eosinophilia is common. Microfilariae are more abundant in blood taken at night, but they can be present at any hour of the day (nonperiodic).
Mansonella streptocerca
M streptocerca occurs in forested areas in Ivory Coast, Ghana, Togo, Benin, Nigeria, Cameroon, Gabon, Central African Republic, Equatorial Guinea, Angola, Democratic Republic of Congo, and Uganda. The primary vector is C grahamii, a day-biting midge. Its geographic distribution overlaps with that of M perstans, O volvulus, and L loa.
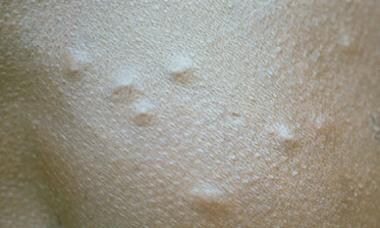
Patients with M streptocerca infection are usually asymptomatic but can develop pruritus, eczema, inguinal adenopathy, and occasional dizziness. The most common presentation is a chronic dermatitis often confined to the chest wall and shoulder where the filaria often lives. The constant scratching may thicken the skin. Patients may also present with hypopigmented macules, 1-3 cm in diameter (see the image below) that resemble tuberculoid leprosy; however, anesthesia is absent. Occasionally, subcutaneous papules may be present on the trunk and shoulders.
A skin reaction, similar to the Mazzotti reaction of onchocerciasis, develops in patients after treatment with diethylcarbamazine (DEC). The skin becomes edematous and intensely itchy, and new cutaneous papules develop around dying microfilariae and adult worms as antigens of the worms become evident to the immune system (see the image below). The inflammatory reaction (composed of eosinophils, neutrophils, and giant cells) attacks these antigens as if they were foreign bodies.
Skin snips taken from people living in areas endemic to M streptocerca may contain microfilariae of O volvulus, M perstans, or L loa along with M streptocerca. Distinction is made by morphologic identification. Skin biopsy specimens taken from the upper trunk and shoulder have the highest frequency of finding M streptocerca.
Mansonella ozzardi
M ozzardi is the only human filarial parasite species that is restricted to the New World. In the West Indies, Suriname, and Argentina, Culicoides species of biting midges are the vectors of M ozzardi. In Brazil and Colombia, blackflies (Simulium amazonicum and Simulium sanguineum) are the vectors. Humans are the only known natural definitive hosts. Prevalence is equal in the sexes and increases with age.
In the West Indies, no specific manifestations of M ozzardi infection are recognized, but patients in the Amazon region attribute a wide variety of complaints to M ozzardi infection, including lymphadenopathy, varices, pain in the knees and ankles, dermatitis, fever, headache, insomnia, and vertigo. Peripheral eosinophilia may be present. No consistent clinical picture has emerged, and these complaints and findings may be incidental.
In biopsy specimens of the skin and subcutaneous tissue, small numbers of microfilariae may be present in blood vessels. Diagnosis depends on identifying microfilariae of M ozzardi in thick or thin films of peripheral blood, skin snips, biopsy specimens of skin, or by Nuclepore filtration of venous blood. The microfilariae are nonperiodic; specimens may be taken at any time of day.
Prevention and treatment of Mansonella infection
Long-term or pulsed treatment with DEC kills the adult M streptocerca worms and should achieve cure. Of note, in a study performed in Zaire (now renamed Democratic Republic of Congo), new papules appeared over a 21-day period of therapy, suggesting that early larval forms of M streptocerca are not susceptible to DEC. Care must be taken if administering DEC to patients co-infected with L loa. Trials with ivermectin reveal that it has strong microfilaricidal activity against M streptocerca. [34] No serious adverse effects are associated, but 45% of patients experienced increased pruritus and acute papular dermatitis after the sixth day of treatment. Single-dose ivermectin (150 μg/kg) has also been shown to reduce microfilariae levels.
For M ozzardi, whereas DEC and albendazole are typically ineffective, ivermectin does have efficacy. [35] In a double-blinded, placebo-controlled study of 40 people with M ozzardi infections in Blanchisseuse, Trinidad, a single dose of ivermectin (6 mg) reduced microfilariae to levels after 4 years by 82.2%. [36] In M ozzardi to date, doxycycline has not been tried to eliminate the symbiotic Wolbachia bacteria.
M perstans has proven difficult to treat. For M perstans, therapy with DEC eliminates the microfilaremia of M perstans infection but only after prolonged therapy. Currently, DEC at 8-10 mg/kg/day for 21 days is recommended, although often not curative. Mebendazole (100-200 mg twice daily for 14-21 days) appears to be more active than DEC in eliminating M perstans infection. Combination treatments using DEC and mebendazole appear to be more effective than DEC alone. Ivermectin and praziquantel are not effective for M perstans infection.
Because M perstans is resistant to standard antiparasitic treatment, doxycycline is sometimes used to eradicate Wolbachia, the endosymbiont found in M perstans as well as most filarial species. Doxycycline treatment typically results in death or sterility of the filarial nematode. In a landmark, open-label, randomized trial, Coulibaly et al recruited patients infected with M perstans from four African villages in Mali. Patients were randomly assigned to receive doxycycline at 200 mg/day orally for 6 weeks (n = 106) or no treatment (n = 110). At 6 months, patients co-infected with W bancrofti underwent a second randomization to receive a single dose of albendazole (400 mg) plus ivermectin (150 mcg/kg) or no treatment. At 12 months, 97% of patients who received doxycycline had no detectable blood levels of M perstans, compared with 16% in the group that did not receive treatment (P<.001). At 36 months, M perstans remained suppressed in 75% of patients who received doxycycline. This suggests that doxycycline is an effective therapy for M perstans infection. [37]
Dirofilariasis
Filarial nematodes of the genus Dirofilaria cause dirofilariasis. They are transmitted via mosquito and Simulium black flies. The number of human dirofilariasis cases reported has increased dramatically in recent years. [38] However, humans are poor hosts for all Dirofilaria species; the worm usually dies before reaching sexual maturity and does not release viable microfilariae. These worms produce an inconspicuous granulomatous reaction in the subcutaneous tissue or a single, small, pulmonary infarct. Of note, all Dirofilaria examined have contained the Wolbachia endosymbiont.
Dimmitis is the most frequent agent of pulmonary dirofilariasis. Although Dimmitis primarily causes lung lesions, it has, on rare occasion caused intra-abdominal infection and even subcutaneous nodules. [39, 40] Most human cutaneous infections are caused by Dtenuis in the Western Hemisphere and Drepens in the Eastern Hemisphere.
Subcutaneous dirofilariasis clinically presents as either a stationary or, less often, a migratory subcutaneous, flesh-colored to erythematous, variably tender nodule. Lesions may be painful and cause burning and itching. A sensation of the worm moving may also be present. Common locations include the head, neck, breasts, arms, legs, or scrotum.
In rare instances, Drepens may migrate from the skin into the circulatory system, reach pulmonary vessels, and cause pulmonary dirofilariasis. Regardless of whether the worm survives to maturity, it almost invariably causes some dermal or subcutaneous injury.
Treatment and prevention of dirofilariasis
The only method of treatment of human dirofilariasis is surgical removal of the lesion or extraction of the worm. (Ivermectin is the treatment of choice for dogs with heartworm.) Extraction is not essential as worms die and often are cleared or sequestered in granulomata without treatment—extraction is useful in preventing further inflammation. Routine methods for avoiding mosquito and fly bites help prevent infection.
-
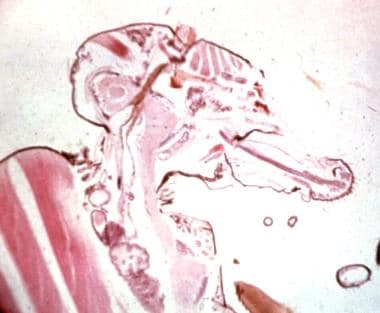
Third stage larvae of Onchocerca in the proboscis of the Simulium black fly.
-
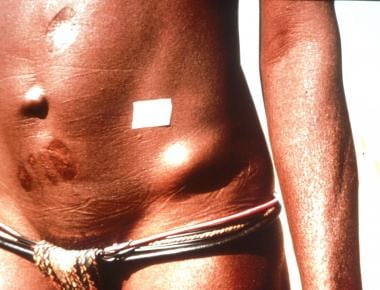
Subcutaneous nodule on hip caused by encysted Onchocerca volvulus.
-
Man blinded by microfilariae of Onchocerca volvulus.
-
Movable, nontender subcutaneous nodule of Onchocerca volvulus.
-
Bleeding on skin from scratching itchy skin of onchodermatitis.
-
One anatomic quarter of body (leg) markedly affected by edema and papule formation of onchodermatitis.
-
Skin with an aged appearance in a patient with chronic onchodermatitis. Note the subcutaneous nodule.
-
Chronic onchodermatitis skin demonstrates a loss of elasticity.
-
Skin of a West African person with leopard spot depigmentation.
-
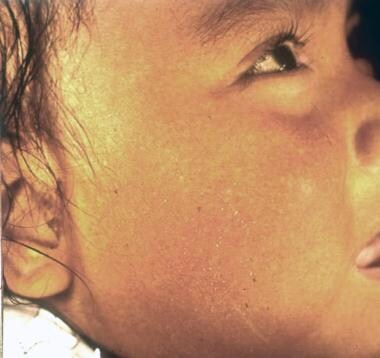
Skin of a Latin American child with erisípela de la costa.
-
Patient from Cameroon with sowdalike lesions.
-
Microfilariae, most numerous in upper dermis.
-
Intraepidermal abscess containing microfilariae of Onchocerca volvulus.
-
Loss of melanin pigment in basal layer of leopard skin.
-
Thin epidermis with a few small rete ridges in elephantoid onchodermatitis.
-
Very thin undulating epidermis lacking rete ridges in a patient with lizard skin onchodermatitis.
-
Hyperkeratosis, focal parakeratosis, and follicular plugging in hyperreactive skin of a patient with sowda onchodermatitis.
-
North American patient with subcutaneous abscess containing zoonotic Onchocerca. Note the very pronounced transverse ridges.
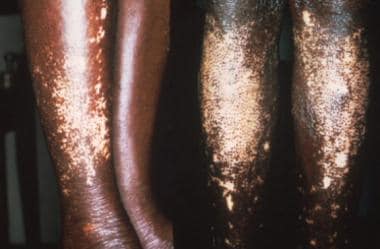
-
Bancroftian filariasis, causing edema (left) and elephantiasis (right).
-
Nodular and hyperplastic changes cause massive distortion to leg of an Indian man with bancroftian filariasis.
-
Hydrocele and “hanging groin” in a patient with bancroftian filariasis.
-
Urine from a patient with bancroftian filariasis demonstrating chyluria.
-
Foot with verrucous skin change in a patient with bancroftian filariasis.
-
Vector for Loa loa, Chrysops, feeding on human skin.
-
Angioedema causing swelling of the face in a woman with Loa loa infection.
-
Focal abscess in hernia sac caused by degenerating adult Mansonella perstans.
-
Microfilariae of Mansonella perstans in peripheral blood smear.
-
Anterior end of microfilariae of Mansonella streptocerca in collagen layers of skin.
-
Posterior end of microfilariae of Mansonella streptocerca in collagen layers of skin.
-
Hypopigmented macules of streptocerciasis.
-
Skin biopsy of Mansonella streptocerca dermatitis.
-
No reaction to healthy adult female Mansonella streptocerca.
-
Abscess formation around degenerating adult Mansonella streptocerca following diethylcarbamazine treatment.
-
Skin reaction to diethylcarbamazine therapy in a patient with streptocerciasis.
-
Microfilariae of Mansonella ozzardi.
-
Cuticle of Dirofilaria (Nochtiella) repens demonstrating thick 3-layered cuticle with prominent external longitudinal ridges. Note the acute inflammatory cells responding to the infection.
-
Biopsy of skin abscess shows a degenerating adult Loa loa in a Calabar swelling.