Background
Human immunodeficiency virus (HIV)–associated lipodystrophy is a syndrome that occurs in HIV-infected patients who are being treated with antiretroviral medications. Antiretroviral therapy (ART) has successfully improved HIV-associated complications and mortality by reducing opportunistic infections. This occurs primarily by decreasing the viral load and increasing the CD4 count. However, metabolic complications and lipodystrophy stemming from long-term ART are concerning and may lead to discontinuation of therapy or regimen substitution.
Because no uniform morphologic changes occur with HIV lipodystrophy, lipohypertrophy and lipoatrophy are considered distinct entities, with different risk factors and metabolic processes underlying their development. Although the term HIV-associated lipodystrophy refers to abnormal central fat accumulation (lipohypertrophy) and localized loss of fat tissue (lipoatrophy), some patients have only lipohypertrophy, some have only lipoatrophy, and, less commonly, a subset of patients exhibits a mixed clinical presentation. [1] This article addresses both lipohypertrophy and lipoatrophy, with a focus on the morphologic changes and underlying pathophysiology of HIV-associated lipodystrophy.
Lipohypertrophy in this syndrome is characterized by the presence of an enlarged dorsocervical fat pad, circumferential expansion of the neck, breast enlargement, and abdominal visceral fat accumulation. Alternatively, lipoatrophy is exemplified by peripheral fat wasting with loss of subcutaneous tissue in the face, arms, legs, and buttocks.
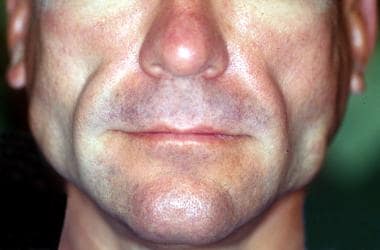
Facial lipoatrophy consists of a progressive loss of facial fat and can be disfiguring cosmetically. Involvement of the face is most common and carries a social stigma and is associated with decreased self-esteem, depression, sexual dysfunction, social isolation, and decreased quality of life; it may also pose a barrier to treatment and reduce medical adherence. [2, 3, 4, 5] Decreased malar fat and temporal fat results in deep skin furrows, and lines of expression become more prominent. This can result in an aged or wasting appearance. [6] This emaciated look creates a stigma of HIV infection and the fear of an unintentional disclosure of HIV status. [6, 7] See the image below.
Although the exact etiology of lipodystrophy is still unknown, both ART and HIV cause changes in lipid distribution. [8] Metabolic abnormalities associated with ART include insulin resistance, hyperlipidemia, and endothelial dysfunction, which can increase the risk of cardiovascular disease. [5] The incidence of diabetes mellitus or atherosclerotic cardiovascular disease is increased secondary to hyperglycemia (from insulin resistance) or hyperlipidemia, respectively. Dietary consideration should be provided to optimize treatment-associated complications. [9] Preventive screening for cardiovascular diseases caused by metabolic alterations should be routinely performed. [10] Lipodystrophy has been shown to be associated with anxiety and poor body image, for which providers should offer referrals for mental health services to those in need. [11] It is important to note that alternative treatments for HIV are emerging, which may address the adverse effects caused by ART and have the potential to replace ART regimens. [12, 13]
The following Medscape articles address other forms of lipodystrophy:
The following Medscape Resource Centers may be helpful as well:
For other discussions on management of HIV infection, see HIV Infection and AIDS, Pediatric HIV Infection, and Antiretroviral Therapy for HIV Infection.
Pathophysiology
Although the precise mechanisms underlying HIV lipodystrophy are not well understood, several hypotheses based on in vitro and human studies may explain the pathogenesis of the lipid changes that take place. Most experts currently believe that HIV type 1 (HIV-1) protease inhibitors (PIs) and nucleoside reverse transcriptase inhibitors (NRTIs), especially stavudine and zidovudine, are implicated. [14, 15, 16, 17, 18] Genetic factors in the patient may confer particular susceptibility. [19, 20] In addition, immunohistochemical components may play a role in the process of lipodystrophy in HIV patients. [21]
One mechanism in which PIs function is by down-regulating PPAR-gamma and C/EBP-alpha, which are key adipogenic transcription factors. Once these factors are down-regulated, there is an interruption with lipogenesis and adipocyte maturation. In addition, they create a large production of reactive oxygen species, which leads to the production of cytokines, macrophage recruitment, and inhibition of glucose transport 4 (GLUT-4), along with a deficiency in insulin signaling and the hormones leptin and adiponectin. [22, 23] If adiponectin is replaced, studies have shown decreased PI toxicity. [24] PIs have also been shown to activate endoplasmic reticulum stress pathways by depleting the calcium in adipocytes. In addition, they also interfere with the expression of regulator genes, CHOP, ATF4, XBP, and GRP78/Bip, which further alters lipid metabolism and autophagy. [22, 25]
PIs also have a high affinity for the catalytic site of HIV-1 protease, which shares a 60% sequence homology with two proteins involved in lipid metabolism, cytoplasmic retinoic acid–binding protein type 1 (CRABP-1) and low-density lipoprotein receptor–related protein (LDLR-RP).
Inhibition of CRABP-1 impairs the production of retinoic acid, leading to decreased fat storage and adipocyte apoptosis, with the subsequent release of lipids into the circulation. Inhibition of LDLR-RP results in hyperlipidemia secondary to the failure of hepatic and endothelial removal of chylomicrons and triglycerides from the circulation.
NRTIs inhibit mitochondrial DNA (mtDNA) polymerase gamma, leading to mtDNA depletion, respiratory chain dysfunction, and reduced energy production, which, in turn, causes insulin resistance and secondary dyslipidemia. [26, 27] Interestingly, mtDNA is depleted only at normal oxygen levels; hypoxic adipocytes do not take up triglycerides and are resistant to mtDNA-induced damage, except after treatment with NRTIs. [28]
Some PIs, particularly ritonavir, inhibit cytochrome P450 3A, a key enzyme in lipid metabolism. Ritonavir has also been shown to cause an extreme amount of apoptosis. On the other hand, atazanavir has been shown to cause apoptosis and autophagy in differentiating adipocytes. [25] The PIs saquinavir, ritonavir, and nelfinavir directly inhibit the development of adipocytes from stem cells and increase the metabolic destruction of fat in existing adipocytes.
Taking genetics into account, a missense mutation in the resistin gene has been shown to have an association with hyperlipidemia, insulin resistance, and limb fat loss when combined with highly active antiretroviral therapy (HAART). [22] Other studies have shown that resistin can function as a useful biomarker for peripheral adipose tissue loss and may be the future of therapeutic strategies. [29] In addition, genetic variants exist in plasma levels, specifically an increased RBP4 and a decreased level of omenti, in patients with HIV-associated lipodystrophy. [30] Also associated is increased circulating fibroblast growth factor 23 (FGF23) levels. [31] Further studies are needed to examine how these updated associations can be incorporated in advancing treatments and early detection of lipodystrophy.
A 2016 study done on rats has shown that PIs reduce the activity of paraoxonase 1 (PON1), which is an high-density lipoprotein–bound esterase that inhibits the decomposing lipid peroxidation products, which further inhibits atherosclerosis. Therefore, there is a higher risk of atherosclerosis and a greater chance of experiencing lipodystrophy. [32]
In observation of immunohistochemical components, a 2014 cross-analytical study analyzed the cytokine expression from adipose tissue obtained in biopsies in 19 HIV patients experiencing lipodystrophy. As a result, tissue necrosis factor (TNF)–alpha and caspase-3 were more prominent in men than in women. In addition, the patients with lipodystrophy had less TNF-beta when being compared with the control group. Lastly, the group of individuals that experienced longer exposure to HIV and HAART had a positive association with levels of TNF-alpha. [21] As shown, sex differences lead toward different pathophysiologic outcomes, but more so, the elevation in cytokine production elevates the likelihood of developing lipodystrophy in HIV patients.
In another 2014 study focused on HIV patients with lipodystrophy, 21% of women and 37% of men were found to have growth hormone deficiencies (GHDs). Men who had GHD had higher amounts of visceral adipose tissue, subcutaneous adipose tissue, and trunk fat. Women who had GHD had significantly lower insulinlike growth factor-1 (IGF-1). Overall, adipose tissue distribution accounts for growth hormone sex differences; those with deficiencies have more problems with lipodystrophy. [33]
Evidence also suggests decreased insulin sensitivity and beta-cell dysfunction in patients with HIV-associated lipodystrophy. [34] Additionally, researchers have found that estrogen receptor expression is down-regulated in the subcutaneous adipose tissue of these patients. This is due to the effects of HAART regimens that include PIs. Stavudine has been particularly implicated in the apoptosis of adipocytes, affecting both dividing and differentiating cells. [35, 36]
In addition, HIV-1 may cause dyslipidemia and lipodystrophy in the absence of HAART, via impaired cholesterol efflux from macrophages and increased TNF-alpha, which modulates free fatty acid metabolism and lipid oxidation and attenuates insulin-mediated suppression of lipolysis. [26, 37] TNF-like weak inducer of apoptosis (TWEAK) is a multifocal cytokine that is decreased in patients with HIV-associated lipodystrophy. [38]
A 2006 study in HIV-positive patients on HAART found that resting energy expenditure and lipid oxidation were significantly higher in those with lipodystrophy than in those without lipodystrophy. [39]
More recent research has found an association with interleukins (ILs) and lipodystrophy, although this association is not completely understood. Findings include lower levels of IL-18 and IL-18 mRNA receptors in skeletal muscle in patients with HIV-associated lipodystrophy. [40] In addition, lower levels of IL-4 and IL-10 are thought to influence the development of lipodystrophy in patients with HIV infection. [41]
Etiology of HIV Lipodystrophy
Part of the early difficulty in establishing the risk factors for HIV-associated lipodystrophy has been agreement on a case definition. Fat accumulation and lipoatrophy are clinically distinct and appear to have separate risk factors. Because most patients are taking a regimen of combined antiretroviral medications, identifying a specific class of antiretroviral associated with lipodystrophy has proved difficult. Despite this, the most common culprits of HIV-associated lipodystrophy appear to be those regimens containing PIs and thymidine analogue NRTIs.
Lipodystrophy associated with PIs occurs 2-12 months after starting PI therapy. Previous reports have shown that ritonavir-saquinavir combinations have a stronger association with abnormal fat accumulation than indinavir or nelfinavir. One study revealed that switching from other PIs to nelfinavir led to an improvement in lipodystrophy symptoms. The association between ritonavir and hypertriglyceridemia is stronger than that with other PIs.
An increased risk of lipodystrophy is reported with the addition of NRTIs (eg, stavudine) to PI treatment compared with treatment with only PIs. Of the NRTIs, the thymidine analogues stavudine (d4T) and zidovudine (ZDV, previously known as AZT) are mostly directly implicated in lipodystrophy, particularly lipoatrophy; switching to a different NRTI such as tenofovir or abacavir can produce demonstrable increases in limb fat and can improve lipid profiles. [42, 43]
In children with HIV, both thymidine analogue NRTIs and PIs are implicated in the development of lipodystrophy. [44] Lipodystrophy has been reported in individuals with HIV infection who have never been treated with PIs; possible mechanisms are noted in Pathophysiology.
Other reported risk factors associated with HIV-associated lipohypertrophy are as follows:
-
Duration of antiretroviral therapy
-
Female sex
-
Higher body fat at onset of HAART
-
Higher triglyceride levels
Other reported risk factors associated with HIV-associated lipoatrophy are as follows:
-
Therapy with thymidine analogue NRTIs (eg, stavudine, zidovudine)
-
Lower pretreatment body mass index at onset of HAART
-
Longer duration of HIV infection
-
White race
Other reported risk factors associated with both lipohypertrophy and lipoatrophy are as follows [45] :
-
Duration of HAART therapy
-
Older age
-
Low CD4 count
Epidemiology
United States statistics
Wide variation exists in the literature regarding the prevalence of HIV lipodystrophy. Various studies show the prevalence rate of this syndrome is 2-60% in all patients who are HIV positive [46, 47] ; a 2007 meta-analysis found a prevalence rate of 14-40% in HIV-positive patients on HAART. [48] In untreated patients with HIV infection, a 4% prevalence rate is reported. The incidence of associated new-onset hypercholesterolemia, hypertriglyceridemia, and hyperglycemia is 24%, 19%, and 5%, respectively.
International statistics
Rates of HIV-associated lipodystrophy vary according to country. [17, 49, 50] A prospective cohort study in England demonstrated a 17% prevalence rate after an 18-month follow-up. Variations in the reported prevalence rates are related to a variety of many factors, including age, genetics, HIV medications, and case definition.
In 2014, among all people living with HIV worldwide, the prevalence of lipodystrophy ranges from 10-80%. [51]
Racial, sexual, and age-related differences in incidence
The risk of lipoatrophy is higher in Whites (5.4 odds ratio) than in Blacks. Women are at a higher risk of lipodystrophy than men (1.9 relative risk). Women are more likely to report fat accumulation in the abdomen and breasts and hypertriglyceridemia, whereas men are more likely to describe fat depletion from the face and extremities, along with hypertension and hypercholesterolemia. [52] Increasing age is a risk factor in the development of this syndrome.
Over the last 12 years, the prevalence of lipodystrophy in HIV-infected men has decreased, yet the prevalence of metabolic syndrome and cardiovascular disease risk has increased. [53]
Prognosis for HIV Lipodystrophy
HIV-associated lipodystrophy progressively worsens as PI therapy is continued, and the discontinuation of PI therapy may result in regression. [54]
To the authors’ knowledge, no studies have been conducted to determine the morbidity and mortality from the body morphologic changes of HIV-associated lipodystrophy per se. However, the insulin resistance and hyperlipidemia are associated with excess morbidity and mortality. The incidences of diabetes mellitus and atherosclerotic cardiovascular disease are increased secondary to hyperglycemia (from insulin resistance) and hyperlipidemia, respectively.
Osteopenia of the lumbar spine may be present in patients with increased visceral fat accumulation.
Dorsocervical fat pad accumulation may result in neck pain and sleep apnea.
Patient Education
For patient education information, see the Sexual Health Center and the Cholesterol Center, as well as HIV/AIDS.
-
Facial HIV-associated lipodystrophy in a patient receiving highly active antiretroviral therapy.