Background
Chromoblastomycosis is a chronic fungal infection of the skin and the subcutaneous tissue caused by traumatic inoculation of a specific group of dematiaceous fungi (usually Fonsecaea pedrosoi, Phialophora verrucosa, Cladosporium carrionii, or Fonsecaea compacta) through the skin. [1, 2]
Several cases of infection by Exophiala species have appeared in the literature. [3] Chromoblastomycosis is classified among the subcutaneous mycoses and is ubiquitous; however, the prevalence is higher in rural populations in countries with a tropical or subtropical climate, such as Madagascar in Africa and Brazil in South America.
Nomenclature
Since its identification in the early 1910s, the name of the disease has been frequently misused to encompass other infections caused by dematiaceous fungi. More recently, the advent of immunosuppressive therapies and diseases brought more confusion because of the identification of new agents and clinical settings. With the introduction of the concept of phaeohyphomycosis by McGinnis in 1983, [4] differentiation among these diseases became more obvious. The features of chromoblastomycosis are distinctive enough to consider chromoblastomycosis an independent clinical entity. The infection should not be confused with mycoses, such as mycetoma or phaeohyphomycosis, caused by other dematiaceous fungi.
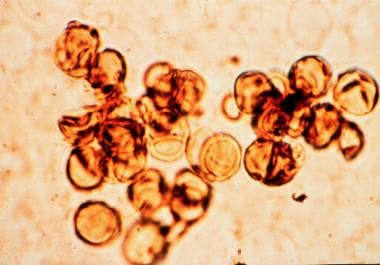
Nowadays, the term chromoblastomycosis is restricted to the cases in which sclerotic cells are present in tissue. Sclerotic cells, also known as Medlar bodies, are globe-shaped, cigar-colored, thick-walled structures that are 4-12 µm in diameter (see the image below).
Medlar first described them in 1915. [5] These structures multiply by septation, and they induce a purulent and granulomatous inflammatory reaction in tissue (see Histologic Findings).
In 1992, [6] the International Society for Human and Animal Mycology (ISHAM) recommended that the best name to define the disease was chromoblastomycosis, which Terra et al coined in 1922. [7]
History
Contrary to what appears in some textbooks, chromoblastomycosis was first described by Max Rudolph in 1914 and not by Lane [8] or Medlar [5] in 1915. In 1987, Castro and Castro [9] reported that Max Rudolph, a German physician living in Brazil, published a preliminary communication where the first 6 cases of the disease were described. Rudolph was also able to isolate a dark-colored fungus from 4 of 6 patients; this fungus grew in culture as a dark grey-to-black–colored furlike colony. Rudolph believed this fungus to be a type of blastomycete, and he successfully inoculated the disease in 4 white rats and 2 monkeys. Surprisingly, he did not describe the histologic aspects of the disease or the pathognomonic sclerotic cells, which both Lane and Medlar described 1 year later.
In 1928, Hoffman [10] mentioned that in Cuba in 1908 Guiteras had observed 10 cases of a disease known as chapa in which the clinical aspects resembled those of chromoblastomycosis. Unfortunately, those cases were not published. In 1920, 2 Brazilian physicians, Pedroso and Gomes, [11] published 4 cases that had been under observation for many years, the first one since 1911. According to them, all 4 cases were caused by P verrucosa. Two years later, in 1922, Brumpt [12] concluded that the agents isolated by Pedroso and Gomes could not be classified as Phialophora species, and he coined the denomination Hormodendrum pedrosoi, later renamed F pedrosoi by Negroni [13] in 1936. By 1930, new cases had been described outside the American continent in France, Sumatra, and Poland. [14]
Four different genera are now widely accepted to cause chromoblastomycosis: F pedrosoi, P verrucosa, C carrionii, and F compacta. [15] Rare cases of chromoblastomycosis caused by Rhinocladiella aquaspersa and Exophiala species have also been reported, allowing the inclusion of these species among those that cause the disease. [16, 17, 18, 19, 20]
Pathophysiology
The infection usually results from a traumatic cutaneous injury that is often not remembered or realized by the patient. The agents often gain entry into the human body by contact with wood splinters or thorns.
The fungi most commonly reported as causing chromoblastomycosis are F pedrosoi, C carrionii, and P verrucosa. A small number of cases due to F compacta, R aquaspersa, and different species of Exophiala have also been reported. In 2007, Chaetomium funicola was identified as a cause of chromoblastomycosis in Panama. [21]
Several authors have demonstrated that a number of different dematiaceous fungi can be isolated from nature. In 1937, Conant [22] demonstrated that a fungus called Cadophora americana, which was isolated from timber, was actually the same organism as P verrucosa. Later isolations were obtained in several countries from materials, such as plants, palm trees, grass, and soil. [19] C carrionii has been isolated from cactaceae, both from the thorns and from the medullae, in Venezuela. [23] C carrionii is the most common agent of chromoblastomycosis in that country, and trauma due to plants is believed to inoculate the skin with the fungus. The fungal cells in human tissue present with the same features as those observed in the inner portions of the plants.
The lesions develop slowly at the site of implantation, producing a warty nodule, which tends to be limited to the skin and the subcutaneous tissue. Over the years, the nodule grows centripetally. In many instances, the central parts of the lesion heal, leaving ivory-colored scars. The disease tends to spread to neighboring healthy skin, forming plaques, which, at times, can involve a whole limb. When nodular lesions predominate over the plaques, the disease assumes a typical cauliflower aspect. Both lymphatic dissemination and cutaneous dissemination have been described.
A new species of Fonsecaea, Fonsecaea nubica, morphologically similar to F pedrosoi and F monophora, has been described in association with chromoblastomycosis. [24] It has described in northern China and in Europe. [25] . Rhinocladiella aquaspersa may also be a causative agent. [26]
Etiology
The isolation of the causative fungi from nature on several different occasions has added to the demonstration that fungi gain entry into the host's body through traumatic inoculation [1] ; this finding also confirmed that populations at risk are rural workers who walk barefoot in endemic regions where the causative agents are found.
A Brazilian study has suggested a link between the occurrence of lesions on the buttocks and the habit of sitting on babaçu (Orbignya phalerata) shells. The authors have failed to isolate the causative fungus from the shells. [27] In 2004, Salgado et al [28] were able to isolate the same F pedrosoi from a thorny bush (Mimosa pudica) and from a chromoblastomycosis patient. The lesion had developed after an accident at the site where those bushes grew.
On the other hand, in 1994, Zeppenfeldt et al [23] demonstrated that certain cactaceae from the Falcon state in Venezuela had fungi not only on their surfaces and thorns but also in the medulla. Not coincidentally, the fungus was identified as C carrionii, which is the most commonly found organism in chromoblastomycosis cases in that country. The histologic characteristics of the fungus in plant tissue resembled that found in humans. Experimental inoculation of C carrionii in goats, performed n Venezuela, demonstrated that although chromoblastomycosis affecting that genus of mammals has never been reported, C carrionii caused cellular reactions in goats identical to those seen in humans at the first stages of infection. [29]
In 1989, Tsuneto et al [30] demonstrated a higher frequency of HLA-A29 in patients with chromoblastomycosis; this finding suggests that genetics might have an influence in the acquisition of the disease.
Epidemiology
Frequency
The disease has been described worldwide, but the incidence is greater in tropical and subtropical regions located between 30° N and 30° S. [31]
An estimated 20% of cases are found in temperate climates, such as in Japan, [32] Canada, [33] Finland, [34, 35] and Romania and the Czech Republic. [14] Chromoblastomycosis most commonly affects male agricultural workers in rural regions of North America, Central America, and South America, but chromoblastomycosis does occur worldwide, including Europe, England, India, South Africa, and Australia. The highest incidence of chromoblastomycosis in the world is in the African country of Madagascar, and a large number of cases have been reported in Japan. A survey in the Brazilian Amazon disclosed an extremely high incidence of chromoblastomycosis (1.6 cases per 10,000 population) in a village called Monte Negro, in the state of Rondonia.
Thirty cases from the Republic of China between 2003 and 2016 were evaluated; patients were predominately male (2.75:1) with a mean age of onset of 65.9 years. Disease duration ranged from 2 months to 20 years. [36]
Countries with the highest number of cases are Madagascar and Brazil. Several different African and Latin American countries, such as Gabon, Colombia, Venezuela, Cuba, the Dominican Republic, and Mexico, also have high prevalence rates.
F pedrosoi infection is most commonly observed in humid climates, whereas C carrionii infection is normally found in dry areas.
Farming is the most common occupation in patients with chromoblastomycosis. [37]
Fonsecaea monophora is the predominant etiologic agent of chromoblastomycosis in southern China. [38]
Chromoblastomycosis in central Kerala, India, was found to be more common among male agriculturists than others. The most common etiologic species isolated was F pedrosoi. [39]
Race
No racial predilection is usually noted. In one Brazilian study in São Paulo State, chromoblastomycosis was evident most in white male rural workers, who averaged age 60 years. [40]
Sex
Most series reports indicate a clear male predominance, although a small number of reports describe similar male-to-female ratios. Approximately 70% of cases are seen in men. The explanation for this male predominance is not clear, but men are assumed to be more commonly involved in agricultural work and are more prone to inflict injuries on themselves, thereby causing self-inoculation, rather than women who are supposedly more dedicated to house jobs.
The possible inhibitory effect of female hormones on the growth of fungi may partially explain the relatively low number of cases in females.
Age
Patients with the disease are most commonly aged 30-50 years. The period between inoculation and disease development is believed to last years, explaining the scarcity of children with chromoblastomycosis. The disease is rarely found in children exposed to the same environmental conditions as adults.
Prognosis
The prognosis for chromoblastomycosis tends to be good, especially for small and localized lesions. When the affected area is large, as in severe cases, cure is difficult, although control is easily achieved. Cicatricial and unaesthetic scars are the rule after the disease is eliminated.
Success in a specialized Brazilian center with clinical care, laboratory support, and pharmaceutical care was noted in 18 cases, with a cure rate of 80%.
Mortality due to chromoblastomycosis is a rare event.
Morbidity relates directly to the severity of the disease. Initially, in the papule or nodule phase, the disease is asymptomatic. When the nodules coalesce, forming large plaques and sometimes involving the whole limb, complications may appear. Common complications include ulceration, lymphedema, and secondary infection.
Fungal causes of chromoblastomycosis are becoming more frequent opportunistic pathogens in solid organ transplant recipients, producing significant morbidity. [41]
-
Sclerotic cells on a potassium hydroxide preparation.
-
Micromorphology of Cladosporium carrionii (left) and Fonsecaea pedrosoi (right), the 2 most commonly isolated agents in chromoblastomycosis.
-
Chromoblastomycosis, tumoral form. Chronic disease led to elephantiasis and involvement of the entire lower limb.
-
Plaque lesion on the foot. The verrucous aspect of the lesion differentiates it from other infectious dermatoses that may present as a verrucous lesion, namely, cutaneous leishmaniasis, sporotrichosis, cutaneous tuberculosis, and cutaneous mycobacteriosis.
-
Culture of Fonsecaea pedrosoi on Sabouraud agar. The black velvety colony has the same macroscopic appearance as the colonies of other chromoblastomycosis-causing agents (eg, Cladosporium carrionii, Fonsecaea compacta, Phialophora verrucosa, Rhinocladiella aquaspersa, Exophiala species).
-
Hematoxylin and eosin–stained section shows typical sclerotic cells inside an abscess. Sclerotic cells present as round, thick-walled, cigar-colored structures.