Practice Essentials
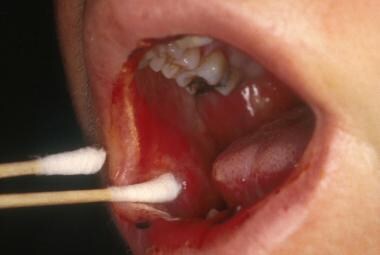
Oral mucositis is a common complication of cancer chemotherapy. It begins 5-10 days after the initiation of chemotherapy and lasts 7-14 days. Chemotherapy-induced oral mucositis causes the mucosal lining of the mouth to atrophy and break down, forming ulcers. See the image below.
See Cancer Chemotherapy: Keys to Diagnosing Common Toxicities, a Critical Images slideshow, to help recognize some of the more common complications of chemotherapy.
Signs and symptoms
Patients typically experience the following:
-
Oral pain and burning
-
Erythema and ulcerations
-
Difficulty eating, drinking, and speaking
-
Difficulty with mouth care regimens
See Clinical Presentation for more detail.
Diagnosis
The diagnosis of chemotherapy-induced oral mucositis is based on clinical findings and the chronology of the development of lesions.
Cultures should be performed if erythema and ulcers are located on the hard palate, attached gingiva, or dorsum of the tongue. Biopsy may be indicated, but it is not routinely necessary for diagnosis.
The two most commonly used scales for grading oral mucositis are the following:
-
World Health Organization (WHO) Oral Toxicity Scale: Combines both objective and functional elements into a single score
-
National Cancer Institute Common Toxicity Criteria (NCI CTC): Scores functional elements only
See Workup for more detail.
Management
The 5 main approaches to managing oral mucositis are as follows:
-
Oral debridement (eg, brushing, toothettes); mucolytic agents, such as Alkalol, help dislodge dried secretions
-
Oral decontamination, including antibacterial and antifungal rinses
-
Prophylaxis, such as ice-chip cryotherapy or Palifermin (keratinocyte growth factor, FDA approved for mucositis prevention in hematopoietic cell transplantation)
-
Photobiomodulation therapy (low-level laser therapy)
A bland, soft diet is recommended. Patients should avoid acidic, spicy, salty, coarse, and dry foods. Patients should keep the mouth moist with frequent sips of water, ice chips, or popsicles. Patients with severe oral mucositis may require total parenteral nutrition.
See Treatment and Medication for more detail.
Consultations
Patients with poorly controlled symptoms and difficulty eating may benefit from consultation with the following services:
-
Oral medicine specialist
-
Pain and palliative care specialist
-
Nutritionist
Patient education
Patients should be informed about their risk for developing oral mucositis, as well as potential signs and symptoms, and should alert their provider at the earliest onset of oral discomfort. Patients should also be educated about the importance of maintaining good oral hygiene throughout the course of their cancer therapy. [3]
Background
Most patients receive chemotherapy on an outpatient basis and are admitted to the hospital if they develop fever and neutropenia, obvious infection, or some other complication. Most of the data cited in this article are from studies performed on patients in an inpatient setting. Nevertheless, oral complications, when they arise in either the inpatient setting or the outpatient setting, are similar.
Chemotherapy, either at conventional levels or in the higher-dosed myeloablative protocols used in conditioning regimens (with or without total body radiation in preparation for hematopoietic cell transplantation [HCT]), often results in erythema, edema, atrophy, and ulceration of the oral mucosa, a condition generally referred to as oral mucositis. Oral mucositis leads to pain and restriction of oral intake, and, in severe cases (eg, patients undergoing myeloablative therapy prior to HCT), necessitates total parenteral nutrition and increased use of narcotic analgesics. [4]
Prospective data on the incidence of severe mucositis during conventional cycled chemotherapy are lacking for various solid and hematologic malignancies; however, Lalla et al, while reviewing published trials for the 2014 Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) guidelines, found that mucositis occurs in approximately 20-40% of patients receiving conventional chemotherapy and in 80% of patients receiving high-dose chemotherapy. [5] It is generally accepted that mucositis is underreported when measured as a toxicity compared with studies in which mucositis is the endpoint being evaluated. In an interventional study by Rosen et al evaluating patients with colorectal cancer being treated with 5-fluorouracil and leucovorin, the incidence of ulcerative mucositis was approximately 50% in the placebo group. [6]
In patients undergoing HCT, oral mucositis is reported as the most debilitating aspect of their treatment. Once present, ulcers may act as a site for local infection and a portal of entry for oral flora that in some instances, may increase the risk of developing septicemia. In addition to direct morbidity, oral mucositis contributes indirectly to increased length of hospitalization and increased cost of treatment.
Targeted therapies
With the introduction of biologically targeted anticancer therapies, emerging oral toxicities have been identified in cancer patients that appear to be distinct from classic mucositis. These toxicities, for the most part, remain poorly described. The class of mammalian target of rapamycin (mTOR) inhibitors, including sirolimus (rapamycin), temsirolimus, and everolimus, are increasingly being used as anticancer agents for neuroendocrine tumors of the gastrointestinal tract, lung, and pancreas and breast and renal cancers, and have been associated with the development of oral aphthous–like ulcers, referred to as mTOR inhibitor–associated stomatitis, or mIAS. [7] They occur in 35-52.9% of patients and are characterized by discrete, ovoid ulcers with a characteristic erythematous halo, and they appear clinically identical to idiopathic aphthous stomatitis in otherwise healthy patients. Like aphthous ulcers, these are confined to the nonkeratinized tissues and have a quicker onset compared with classic mucositis.
Because this toxicity clusters with dermatologic toxicities, rather than GI (as is the case with conventional mucositis) toxicities, this suggests that the underlying pathophysiology is likely very different. Investigations from 2017 suggest the following three-stage sequence [8, 9] :
- Direct mTOR inhibitor–initiated epithelial injury
- Release of proinflammatory cytokines
- Activation of the innate immune response with influx of inflammatory cells
Treatment approaches known to be effective for the management of aphthous stomatitis, such as high-potency topical steroids, also may be effective for mIAS. [8, 9]
Dysgeusia and xerostomia have been reported adverse effects in these targeted therapies. They tend to have a greater impact on quality of life; however, these adverse effects may also affect the patients ability to maintain adequate nutritional intake, leading to weight loss.
Pathophysiology
Oral mucositis results from a complex interaction of local tissue damage, the local oral environment, the patient's level of myelosuppression, and the patient's intrinsic genetic predisposition (eg, single nucleotide polymorphisms) to develop oral mucositis.
The current working biological model for oral mucositis is based on five interrelated phases, including an initiation phase, a message-generation phase, a message-activation and up-regulation phase, a signaling and amplification phase, and an ulceration/microbiological phase, and a healing phase. [10] In the initiation phase, cellular damage causes direct DNA breaks with the generation of reactive oxygen species, which damage lipids, DNA, and connective tissue, leading to cell death and the release of inflammatory substances. In the up-regulation/activation phase, transcription factors such as nuclear factor (NF)–κβ and distinct pathogen-associated molecular patterns such as toll-like receptor signaling emerge.
During the signaling and amplification phase, positive feedback loops are activated. For example, tumor necrosis factor (TNF)–α activates NF-κβ, mitogen-activated protein kinase (MAPK), and sphingomyelinase pathways, while also contributing directly to cellular and tissue injury. The result is erythema from increased vascularity and epithelial atrophy 4-5 days after the initiation of chemotherapy. Microtrauma from day-to-day activities, such as speech, swallowing, and mastication, leads to ulceration.
During the ensuing ulcerative/bacteriologic phase (during which time neutropenia is common), putative bacterial colonization of ulcerations occurs, resulting in the flow of endotoxins into mucosal tissues and the subsequent release of more interleukin (IL)–1 and TNF-α. This is likely the phase most responsible for the clinical pain and morbidity associated with oral mucositis.
During the fifth and final healing phase, cell proliferation occurs with reepithelialization of ulcers. Signals from the extracellular matrix induce epithelial cells to migrate underneath the pseudomembrane (fibrin clot) of the ulcer. The epithelium then proliferates so that the thickness of the mucosa returns to normal. Reconstitution of the WBCs in neutropenic patients effects local control of bacteria, which also contributes to resolution of the ulcers. However, the direct relationship between the WBC count and oral mucositis is uncertain, and not all patients with mucositis demonstrate hematologic toxicity.
Etiology
The underlying malignancy and the intensity and duration of the chemotherapy regimen are the two most important factors in determining the occurrence and the severity of oral mucositis. Hematologic malignancies and highly myelotoxic regimens are typically associated with more severe oral mucositis, but many factors can modify the occurrence and the degree of oral mucositis.
Other factors that modify the occurrence and the severity of oral mucositis include level of pretreatment oral health, oral care during treatment, and salivary flow. Poor oral health before and during treatment and hyposalivation all contribute to an increased risk and increased severity of mucositis. The use of methotrexate for graft versus host disease (GVHD) prophylaxis is an additional significant risk factor for oral mucositis, and the use of non–methotrexate-containing regimens has been shown to reduce the overall severity of mucositis. [11] Stomatitis occurs more frequently in patients with breast cancer treated with a 5-flurouracil (5-FU), doxorubicin, and cyclophosphamide regimen versus a doxorubicin and paclitaxel regimen. [12] Nevertheless, other factors, including underlying genetic predisposition, likely also play an important role in determining risk.
Generally, patients with hematologic malignancies have an increased rate of oral mucositis compared with those with solid tumors. This is, to some extent, related to the treatment regimens.
Great variability exists in the stomatotoxicity of different treatment regimens. Some of the most stomatotoxic agents include the antimetabolites 5-fluorouracil, methotrexate, and cytarabine.
Concomitant radiation therapy (to the head and neck region) increases the risk of oral mucositis because of synergistic effects with the chemotherapeutic agents.
Chronic irritation from ill-fitting prostheses or faulty restorations predisposes patients to the development of oral mucositis due to local irritation and trauma.
Hyposalivation prior to and during treatment is associated with an increased risk of oral mucositis.
Oral mucositis occurs independently of oral mucosal infections of viral and fungal etiology, but it may be exacerbated by such concomitant infections.
Better pretreatment oral health is probably associated with a reduced incidence of and less severe oral mucositis; however, this has never been proven. Regardless, maintaining good oral hygiene with daily mouth care is important.
Epidemiology
Some degree of oral mucositis occurs in approximately 40% of patients who receive cancer chemotherapy. At least 75% of patients who receive myeloablative conditioning regimens (chemotherapy with or without total body irradiation) in preparation for HCT develop oral mucositis; the incidence may be even higher in children. [13] The incidence is also higher in patients who receive continuous infusion therapy for breast and colon cancer and in those who receive adjuvant therapy for head and neck tumors. However, in patients of the same age with similar diagnoses and treatment regimens and equivalent oral health status, the incidence of oral mucositis may vary considerably. This is most likely because of genetic differences and other factors that are not yet fully characterized or understood.
No racial or sexual predilections are apparent for chemotherapy-induced oral mucositis.
Younger patients tend to develop oral mucositis more often than older patients being treated for the same malignancy with the same regimen. This is apparently because of the more rapid rate of basal cell turnover noted in children, although this phenomenon remains poorly characterized. However, the healing of oral mucositis also appears to occur more rapidly in the younger age group.
Prognosis
Chemotherapy-induced oral mucositis is a self-limiting condition.
Oral mucositis causes pain, restricts oral intake, frequently contributes to interruption of therapy, may increase the use of antibiotics and narcotics, may increase the length of hospitalization, and may increase the overall cost of treatment. Patients with oral mucositis and neutropenia have a relative risk of septicemia more than four times that of patients with neutropenia without oral mucositis.
Patients with pulpal disease from dental caries or trauma, periodontal disease, and low-grade soft-tissue infections (especially those associated with partially erupted third molars) are at increased risk for bacteremia due to oral viridans streptococci (Streptococcus mitis, Streptococcus oralis, Streptococcus acidominimus); the rate was reported to be as high as 28% in subjects undergoing HCT. All of these patients had either gingivitis or periodontitis. [14]
Oral mucositis lesions have been implicated as an important portal of entry for these organisms into the systemic circulation because many of these organisms are native to the oropharyngeal region. Combination prophylaxis, including the use of penicillin and other antibiotics effective against gram-positive streptococci, has been effective in reducing the incidence of septicemia.
-
Hairy tongue.
-
Multiple mucoceles on the hard palate.
-
Erythematous oral mucositis lesion on the buccal mucosa.
-
Ulcerative oral mucositis lesion on the buccal mucosa.
-
Ulcerative oral mucositis lesion on the lateral and ventral surfaces of the tongue.
-
Ulcerative oral mucositis lesions on the labial mucosa and the floor of the mouth.
-
Oral pseudomembranous candidiasis on the hard palate.
-
Herpes simplex virus ulceration on the dorsal surface of the tongue.
-
Herpes simplex virus ulceration on the hard and soft palate. Note lesions on the right upper lip and the dorsum of the tongue.
-
Acute graft versus host disease involving the dorsal surface of the tongue. This is a keratinized site that is usually not involved by oral mucositis.