Background
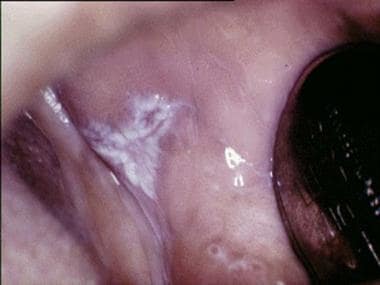
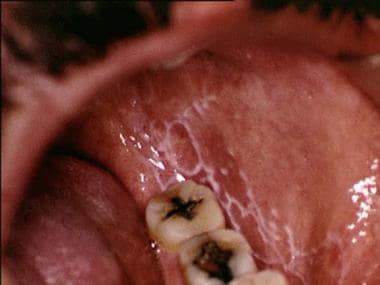
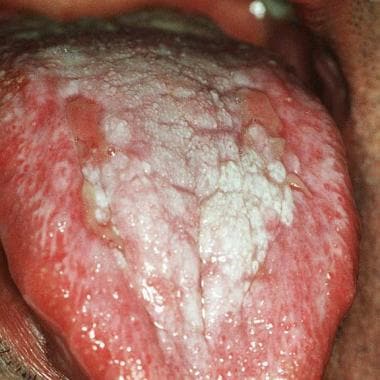
Oral lichen planus (OLP) is a chronic mucocutaneous disorder that presents in a wide range of clinical forms, such as unilateral or bilateral white striations, papules, or plaques on the buccal mucosa, labial mucosa, tongue, and gingiva. Erythema, erosion, and blisters may or may not be present. Note the images below.
Pathophysiology
Current data suggest that oral lichen planus (OLP) is a T-cell–mediated autoimmune disease in which autocytotoxic CD8+ T cells trigger apoptosis of oral epithelial cells. [1, 2, 3]
The dense subepithelial mononuclear infiltrate in oral lichen planus is composed of T cells and macrophages, and there are increased numbers of intraepithelial T cells. Most T cells in the epithelium and adjacent to the damaged basal keratinocytes are activated CD8+ lymphocytes. Therefore, early in the formation of oral lichen planus lesions, CD8+ T cells may recognize an antigen associated with the major histocompatibility complex (MHC) class I on keratinocytes. After antigen recognition and activation, CD8+ cytotoxic T cells may trigger keratinocyte apoptosis. Activated CD8+ T cells (and possibly keratinocytes) may release cytokines that attract additional lymphocytes into the developing lesion. [2]
Oral lichen planus lesions contain increased levels of the cytokine tumor necrosis factor (TNF)–alpha. [4, 5] Basal keratinocytes and T cells in the subepithelial infiltrate express TNF in situ. [6, 7] Keratinocytes and lymphocytes in cutaneous lichen planus express elevated levels of the p55 TNF receptor, TNF-RI. [8] T cells in oral lichen planus contain mRNA for TNF and secrete TNF in vitro. [9] Serum and salivary TNF levels are elevated in oral lichen planus patients. [10, 11, 12, 13] TNF polymorphisms have been identified in patients with oral lichen planus, and they may contribute to the development of additional cutaneous lesions. [14] Oral lichen planus has been treated successfully with thalidomide, [15, 16] a drug known to suppress TNF production. [17, 18] Together, these data implicate TNF in the pathogenesis of oral lichen planus.
Of possible significance, elevated concentrations of interleukin-6 (IL-6) and neopterin in saliva and serum of patients with the erosive-atrophic form of oral lichen planus suggest they may be involved in the etiology of this disease variation. [19] Research published in 2015 also suggests that osteopontin, CD44, and survivin may be involved in the pathogenesis of oral lichen planus. [20] Additionally, microRNA 4484 (miR-4484) has been found to be significantly up-regulated in the salivary exosomes of patients with oral lichen planus. [21]
The specific antigen that triggers lichen planus is unknown, although it may be a self-peptide (or altered self-peptide), in which case lichen planus would be a true autoimmune disease. The role of autoimmunity in the pathogenesis is supported by many autoimmune features of oral lichen planus, including its chronicity, onset in adults, predilection for females, association with other autoimmune diseases, occasional tissue-type associations, depressed immune-suppressor activity in patients with oral lichen planus, and the presence of autocytotoxic T-cell clones in lichen planus lesions. The expression or unmasking of the lichen planus antigen may be induced by drugs (lichenoid drug reaction), contact allergens in dental restorative materials or toothpastes (contact hypersensitivity reaction), mechanical trauma (Koebner phenomenon), viral infection, or other unidentified agents. [22, 23, 24]
Etiology
Current data suggest that oral lichen planus (OLP) is a T-cell–mediated autoimmune disease in which autocytotoxic CD8+ T cells trigger the apoptosis of oral epithelial cells. However, the precise cause of oral lichen planus is unknown.
Reported associations between oral lichen planus and systemic diseases may be coincidental, because (1) oral lichen planus is relatively common, (2) oral lichen planus occurs predominantly in older adults, and (3) many drugs used in the treatment of systemic diseases trigger the development of oral lichenoid lesions as an adverse effect.
In many patients, a cause for the oral lichenoid lesions cannot be identified; in these patients, the disease is called idiopathic oral lichen planus.
Oral lichenoid drug reactions may be triggered by systemic drugs including NSAIDs, beta-blockers, sulfonylureas, some ACE inhibitors, and some antimalarials. In patients with oral lichenoid lesions, be alert for any systemic drug as a cause.
Oral lichenoid contact-sensitivity reactions may be triggered by contact allergens including dental amalgam composite resin and toothpaste flavorings, especially cinnamates. Skin patch testing may help in identifying contact allergens (see Other Tests). If an allergy is detected, lesions may heal when the offending material is removed.
Oral lichenoid lesions may be triggered by mechanical trauma (Koebner phenomenon) from calculus deposits, sharp teeth, rough surfaces of dental restorations or prostheses, cheek or tongue biting, and oral surgical procedures. Scale any teeth associated with oral lichen planus lesions to remove calculus deposits and reduce sharp edges. Dental restorations and prostheses that are associated with oral lichen planus lesions should be mirror-polished.
Some studies show an increased incidence of Candida albicans infection in oral lichen planus. A causal role for C albicans infection in oral lichen planus has not been identified.
Some studies have revealed a prevalence of viral infections in oral lichen planus (eg, hepatitis C virus [HCV]). Some studies report human papillomavirus (HPV) types 6, 11, 16, or 18. [25, 26] Dysplastic oral lichen planus lesions had a higher prevalence of HPV-16 compared with nondysplastic oral lichen planus lesions. Some study findings suggest an association between oral lichen planus and chronic hepatic diseases such as HCV infection, autoimmune chronic active hepatitis, and primary biliary cirrhosis. [27, 28] This association probably reflects the geographic distribution of HCV disease and lichenoid reactions to various drug therapies (eg, interferon-alfa for HCV disease, penicillamine for primary biliary cirrhosis). Oral lichen planus is associated with HCV infection and liver disease in parts of Japan and southern Europe. An association between oral lichen planus and HCV infection has not been detected in British, French, German, Scandinavian, or American patients.
Oral lichenoid lesions may arise in people who habitually chew betel quid. A causal role for betel quid in oral lichen planus has not been identified.
Oral lichenoid lesions are part of the spectrum of chronic graft versus host disease that occurs after allogeneic hemopoietic stem cell transplantation.
No consistent association with human leukocyte antigen (HLA) is reported in oral lichen planus. This finding suggests that the patient's genetic background does not play a critical role in oral lichen planus pathogenesis.
Exacerbations of oral lichen planus have been linked to periods of psychological stress and anxiety. [29, 30]
Little evidence supports a connection between diabetes mellitus and oral lichen planus. The oral lichenoid lesion in Grinspan syndrome (triad of oral lichen planus, diabetes mellitus, and hypertension) is probably an adverse effect of the drug therapy for diabetes mellitus and hypertension.
Several good reviews are available on the etiopathogenesis of oral lichen planus. [31, 32]
Epidemiology
Frequency
Oral lichen planus (OLP) affects approximately 1-3% of the general adult population, although the prevalence of the disease is unknown in many areas. [33]
Race
Oral lichen planus affects all racial groups.
Sex
The female-to-male ratio for oral lichen planus is 1.4:1.
Age
Oral lichen planus predominantly occurs in middle-aged adults. Younger adults and children can be affected, but rarely. [34]
Prognosis
Oral lichen planus (OLP) is a mucosal subtype of lichen planus. The lesions of cutaneous lichen planus typically resolve within 1-2 years, whereas the reticular forms of oral lichen planus have a mean duration of 5 years and erosive lesions of oral lichen planus are long-lasting and persist for up to 15-20 years or longer. Resolution of the white striations, plaques, or papules is rare. Symptomatic oral lichen planus (ie, atrophic or erosive disease) characteristically waxes and wanes, although the associated white patches rarely resolve. Patients with atrophic (erythematous) or erosive (ulcerative) disease commonly have significant local morbidity. This condition can be aggravated by stress and can have a significant negative impact on quality of life. [35]
Current immunosuppressive therapies usually control oral mucosal erythema, ulceration, and symptoms in patients with oral lichen planus with minimal adverse effects. However, a range of therapies may need to be tried.
Advise patients that oral lichen planus lesions may persist for many years, with periods of exacerbation and quiescence.
Follow up patients with oral lichen planus at least every 6 months for clinical examination and repeat biopsy as required, although patients should be advised to seek medical care whenever the symptoms are exacerbated or the presentation of the lesions change. This lesion can have a potential malignant transformation at a rate of 0.04-1.74% as reported in the literature, although there is controversy regarding the etiopathogenesis of this transformation. Careful, regular, and long-term follow-up is essential for early detection of malignant transformation. [36]
In the context of appropriate medical care, the prognosis for most patients with oral lichen planus is excellent; it typically is well controlled by topical or systemic therapies.
Patient Education
Patient education is important. Many patients with oral lichen planus (OLP) are concerned about the possibilities of its malignancy and contagiousness. Many patients are frustrated by the lack of available patient education concerning oral lichen planus. [37]
Inform patients with oral lichen planus of the following:
-
The chronicity of oral lichen planus and the expected periods of exacerbation and quiescence
-
The aims of treatment, specifically the elimination of mucosal erythema, ulceration, pain, and sensitivity
-
The lack of large randomized controlled therapeutic clinical trials
-
The possibility that several treatments may need to be tried
-
The potentially increased risk of oral cancer
-
The possibility of reducing the risk of oral cancer (see Complications)
Information about oral lichen planus is currently available online. For instance, an oral lichen planus chat room is available at the homepage of the International Lichen Planus Support Group Web.
For patient education material, visit the Cancer Center, as well as Cancer of the Mouth and Throat.
-
Plaquelike oral lichen planus on the buccal mucosa on the left side.
-
Reticular oral lichen planus on the buccal mucosa on the left side.
-
Ulcerative oral lichen planus on the dorsum of the tongue.
-
Lichen planus nail involvement.