Practice Essentials
This article focuses on noncandidal oral fungal infections (deep mycoses). Candidiasis (candidosis) is by far the most common fungal infection of the mouth (oral cavity). Other Medscape articles on candidiasis include Chronic Mucocutaneous Candidiasis, Mucosal Candidiasis, and Cutaneous Candidiasis.
In rare cases, mycoses can produce clinical disease in healthy persons, including oral lesions. Systemic mycoses in healthy individuals are more common in endemic areas than elsewhere, and they are often asymptomatic and may spontaneously resolve. In otherwise healthy persons, acute pulmonary and primary mucocutaneous symptomatic lesions may resolve without treatment. However, chronic pulmonary infection tends to progress and disseminated infections can be fatal.
Noncandidal fungal infections have the potential for serious injury to the oral cavity, perioral tissues, and, sometimes, the paranasal sinuses, orbit, and cranial base. Orofacial lesions caused by the main systemic mycoses may occasionally be seen in isolation, but they are typically associated with lesions elsewhere, mainly in the respiratory tract. The oral lesions associated with these deep fungal infections are chronic and progress to form solitary, chronic deep ulcers with the potential for local destruction and invasion and systemic dissemination. They can mimic other infections and malignant neoplasms.
Chronic oral ulceration, chronic maxillary sinus infection, or bizarre mouth lesions, especially in patients with HIV disease, oral cancer survivors, [1] patients with SARS-CoV-2 (coronavirus disease-2019), [2] those with lymphoproliferative disorders, persons with diabetes mellitus, or those who have been in endemic areas, may suggest the diagnosis and patients should be treated in consultation with a physician with appropriate expertise.
Most of these mycoses are diagnosed on the basis of a history of foreign travel or an immunocompromised state. Investigations include smears, biopsy, staining with periodic acid-Schiff (PAS) or Gomori methenamine silver, culture of the affected tissues, polymerase chain reaction (PCR), serodiagnosis (sometimes), physical examination, and chest radiography. Definitive diagnosis is achieved by means of microbiologic or histologic identification and serodiagnosis. DNA probes are available for several species. Unfortunately, specific laboratory studies for an accurate diagnosis of many mycoses is available only in a few laboratories.
Prompt identification and treatment, usually with systemic antifungal drugs, are essential; delayed treatment or no treatment can result in considerable orofacial destruction, systemic dissemination, or death.
It is important to highlight that these organisms usually invade other organs as a primary site of infection. [3] Lesions in the oral cavity may be the result of hematogenous spread of the disease; therefore, a diagnosis of a deep-seated fungal oral infection should prompt the clinician to investigate systemic involvement as well as to determine the integrity of the immune system of the affected individual.
Signs and symptoms
Patients with deep mycoses may present with a primary infection of the oral mucosa, but, more commonly, they present with an extension of an established paranasal infection. Therefore, by the time oral lesions are present, considerable destruction of the maxilla and maxillary sinus may have occurred.
In healthy individuals, the disease is usually self-limiting, but in individuals who are immunocompromised, extensive local destruction, fungemia, visceral and cerebral invasion, and death are substantial risks.
The most common presentation of oral deep fungal infection is a chronic, solitary ulcer or nodule. When infection involves the palate, this finding may be only the initial indication of considerable antecedent destruction of the maxilla and maxillary sinus. Extension and/or invasion into the orbital and cranial cavity are not uncommon. The condition may be indistinguishable from other causes of chronic oral ulcers (eg, tuberculosis, malignancy).
Aspergillosis
Orofacial lesions caused by Aspergillus species include antral aspergilloma, invasive aspergillosis of the antrum, indolent chronic sinusitis, allergic sinusitis, and oral lesions. [4, 5, 6] Aspergilloma of the maxillary antrum is uncommon and typically occurs in a healthy host as a hyphal ball in a chronically obstructed sinus.
Invasive sinus aspergillosis is rare and affects mainly immunocompromised hosts, although it is also seen in some apparently healthy individuals, predominantly in subtropical countries with a warm climate (eg, Sudan, Saudi Arabia, India). Patients with coronavirus disease-2019 (COVID-19), [2] leukemia, lymphoma, HIV disease, or iatrogenic immunosuppression (eg, those undergoing bone marrow or renal transplantation) are at particular risk from such invasive sinus aspergillosis. Although A fumigatus is the usual cause of invasive sinus aspergillosis, A flavus appears to predominate in immunocompromised individuals. Rarely, other species (eg, A repens) are encountered, sometimes with other mycoses such as Microascus cinereus.
In invasive sinus aspergillosis, the antral wall is destroyed, with antral pain, swelling, sequelae from orbital invasion (eg, impaired ocular motility, exophthalmos, or impaired vision), or intracranial extension (eg, headaches, meningism).
Chronic sinus aspergillosis is uncommon, and patients present with a diffusely opaque antrum radiographically, sometimes with dense punctate radiopacities. This disease is unresponsive to treatments used for bacterial sinusitis. Allergic fungal sinusitis is also uncommon and is usually due to fungi other than Aspergillus organisms.
Interestingly, subclinical defects in cell-mediated immune responses to Aspergillus species have been observed in patients with sinus aspergillosis. Occasional cases of sinus aspergillosis arise as a result of metastasis from pulmonary aspergillosis or iatrogenic factors following dental procedures such as extractions, endodontics, or implants in the maxilla. [7, 8]
Oral lesions of aspergillosis are seen predominantly in some immunocompromised patients with invasive aspergillosis. Yellow or black necrotic ulcers typically appear on the palate or occasionally on the posterior tongue.
Note the image below.
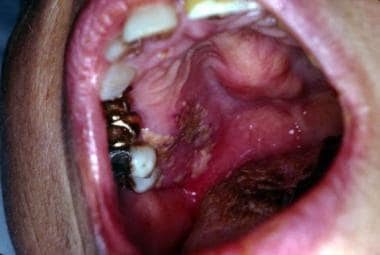 Aspergillosis. Note deep mucosal ulceration and nodular expansion of the hard palate. Courtesy of David Sirois, DMD, PhD.
Aspergillosis. Note deep mucosal ulceration and nodular expansion of the hard palate. Courtesy of David Sirois, DMD, PhD.
Blastomycosis
Blastomycosis [9] may become disseminated to produce ulcerating lesions that affect the oral mucosa. Mandibular involvement is rare. Cutaneous blastomycosis may spread to affect the lips.
Note the image below.
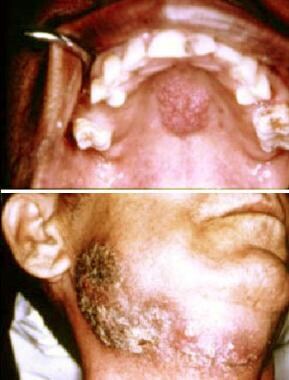 Blastomycosis. Top image shows nonspecific papillary nodular lesion on the hard palate. Bottom image shows extensive ulceration involving the skin of the face and neck. Courtesy of David Sirois, DMD, PhD.
Blastomycosis. Top image shows nonspecific papillary nodular lesion on the hard palate. Bottom image shows extensive ulceration involving the skin of the face and neck. Courtesy of David Sirois, DMD, PhD.
Coccidioidomycosis
Oral lesions from coccidioidomycosis are rare but, when they occur, they typically are reported secondary to lung involvement. They are usually verrucous lesions, sometimes occurring with infection of the jaw. Parotid involvement has been recorded.
Cryptococcosis
Oral cryptococcal infection manifests mainly as nonhealing extraction wounds or chronic ulceration on the palate or tongue. [10] Several cases of cryptococcal oral lesions have been reported, mainly in persons infected with HIV. Rare cases have been reported mainly in individuals with leukemia.
Histoplasmosis
Oral lesions of H capsulatum infection have been recorded mainly in persons with pulmonary or disseminated histoplasmosis, especially in patients with HIV infection. [11] Oral lesions are sometimes isolated and have also been recorded in apparently healthy persons. Oral lesions are usually ulcerative or nodular; have been found on the tongue, palate, buccal mucosa, or gingiva; and rarely invade the mandible or maxilla. [12, 13] Oral lesions in African histoplasmosis are generally localized, affecting the tongue, buccal mucosa, or jaws.
Note the image below.
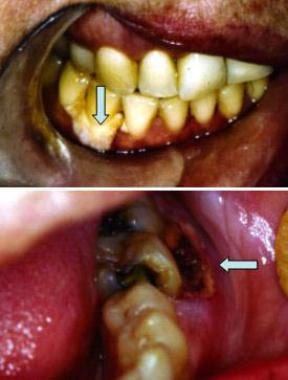 Histoplasmosis. Top image shows periodontal recession and deep ulceration with exposed necrotic alveolar bone (arrow). Bottom image shows solitary, deep ulceration of the gingivae also associated with necrotic bone (arrow). Courtesy of David Sirois, DMD, PhD.
Histoplasmosis. Top image shows periodontal recession and deep ulceration with exposed necrotic alveolar bone (arrow). Bottom image shows solitary, deep ulceration of the gingivae also associated with necrotic bone (arrow). Courtesy of David Sirois, DMD, PhD.
Paracoccidioidomycosis
Oral lesions of paracoccidioidomycosis are chronic, often granular or exophytic, and ulcerated. [14] Antral lesions are rare. Oral lesions of paracoccidioidomycosis appear to be uncommon in persons with HIV disease, although involvement of the submandibular lymph nodes and the presence of lesions outside the head and neck have been reported.
Rhinosporidiosis
Oral lesions of rhinosporidiosis are usually proliferative lumps, especially affecting the soft palate.
Zygomycosis
Rhinocerebral zygomycosis is usually caused by Rhizopus oryzae or Rhizopus arrhizus. This condition has been reported in association with COVID-19 infection. [2] The disease typically commences in the nasal cavity or paranasal sinuses and causes pain, nasal discharge, and fever; the organisms may then invade the palate to produce black, necrotic oral ulcers. Orbital invasion may produce orbital cellulitis, impaired ocular movements, proptosis, and ptosis. Intracranial invasion follows penetration of ophthalmic vessels or the cribriform plate.
The most frequent manifestation in the oral cavity is the development of white or black palatal ulcers, which become necrotic with well-defined borders. They may form rapidly when associated with diabetic decompensation or neutropenia. It is usually an acute condition during which palatal ulcers develop within the first 10 days; therefore, it is important to make an early diagnosis of the disease. The cure rate in the series reported by Bonifaz et al was 19%. [15]
Geotrichosis
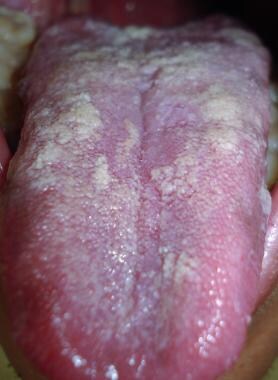
Oral geotrichosis presents as pseudomembranous plaques with an underlying chronic inflammatory reaction, which can resemble oral candidiasis. Ulcerative and hyperplastic variants have also been described. [3] See the image below.
Rhodotorula infection
Oral presentations include nonhealing ulcers and white plaques, which may resemble those of candidiasis. [16]
Fusariosis
Oral lesions occur as secondary lesions derived from blood-borne organisms in disseminated disease. Risk factors include low count of T lymphocytes, neutropenia, and previous fungal infections. It presents as necrotic ulcerations of the gingiva, palate, or tongue. [17]
Diagnostics
Tests can include cytology smears; biopsy and staining with periodic acid-Schiff (PAS) or Gomori methenamine silver stain; cultures; DNA probing, which is becoming increasingly available but at present is not sufficient alone; and, in some circumstances, serodiagnosis, which is available for aspergillosis (galactomannan antigen testing) and cryptococcosis (capsular polysaccharide antigen testing). However, serodiagnosis is not adequate without the other tests mentioned, which are confirmatory tests.
General laboratory testing can include the following:
-
Comprehensive metabolic panel (eg, to evaluate for diabetes or malnutrition)
-
CBC count with differential (eg, to evaluate for neutropenia or lymphopenia)
-
HIV serostatus testing
-
Immunologic testing
The diagnosis of aspergillosis is confirmed by smear results and lesional microscopy findings. Staining with PAS or Gomori methenamine silver shows regular, narrow (4 µm wide), branching, and septate hyphae of Aspergillus species. However, hyphae of Fusarium species, Mucor species, and Pseudallescheria boydii may cause confusion. Immunostains may help for definitive diagnosis. Culture of tissue or fluids on Sabouraud or Mycosel agar may return positive results, but this is not invariable. Furthermore, the organisms are ubiquitous; therefore, isolation of Aspergillus organisms is not proof of disease. Estimations of serum precipitin and immunoglobulin E–specific antibody levels may support the diagnosis.
The diagnosis of blastomycosis is based on smear or culture results. Biopsy may show Blastomyces species in the tissue, with granulomas. Staining with PAS, methenamine silver nitrate, or Fontana-Masson stain helps in the identification. The organism can be mistaken for H capsulatum, C neoformans, Bipolaris species, or Wangiella dermatitidis. Direct immunostaining is the most useful means of confirmation. DNA probes can yield an answer in 2 hours. Skin test and serology results are, unfortunately, unreliable, although serotesting has improved.
Coccidioidomycosis is diagnosed mainly by means of a thorough history and physical examination supported by histology findings that show granulomas with spherules containing endospores. DNA probes are now available. Also helpful are serology and the Spherulin or coccidioidin skin tests. Culture, although useful, is potentially hazardous to laboratory staff.
Cryptococcosis is confirmed by microscopy or by staining with PAS, mucicarmine, or methenamine silver stain. The stains show granulomas. Smears may also be stained with India ink or nigrosin. The yeast cell is round or elliptical, 4-6 µm in diameter, clear, and capsulated. It also fluoresces in ultraviolet light. Culture and assay of serum or cerebrospinal fluid for capsular antigen and antibody (latex agglutination test) may help in diagnosis.
Histoplasmosis is confirmed with microscopy, which shows granulomas with PAS-positive spores with a narrow halo in macrophages and microabscesses with necrosis. DNA probes are now available. Culture results on Sabouraud agar are also confirmatory. Complement fixation tests may be of value; a titer greater than 1:32 indicates infection, but several other serological tests are also available. The histoplasmin skin test result is of little importance in diagnosis.
A diagnosis of mucormycosis is confirmed with smears or the histologic demonstration of tissue invasion by broad (5-50 µm), irregularly wide, nonseptate, branching hyphae. These hyphae characteristically invade blood vessels and cause thrombosis, ischemia, infarction, and necrosis. Methenamine silver or PAS staining is best to show the fungi, which are difficult to culture from a swab sample.
Paracoccidioidomycosis is diagnosed using pus or scrapings from a lesion. Examined in potassium hydroxide, the samples may show the rounded, refractive cells of P brasiliensis, which have characteristic multiple budding. Biopsy is required for definitive diagnosis; it reveals suppurative granulomas with giant cells and blastospores, which are double-contoured, cystlike structures approximately 30 µm in diameter. These are often surrounded by daughter spores (Mickey Mouse appearance). Methenamine silver nitrate or PAS stain is particularly useful to demonstrate the organisms, and direct immunostaining with Blastomyces antiserum is less frequently used.
Cytological examination, although rapid and available, [18] is usually complemented by other investigations. Smear or culture can also be diagnostically useful, but P brasiliensis grows extremely slowly. Serologic examination by means of immunodiffusion, or particularly complement fixation, is useful in epidemiologic studies. Reliable skin tests are not available.
For the diagnosis of geotrichosis, direct examination may provide a rapid diagnosis when multiple hyphae and conidia are present. However, conidia may have features similar to those of Candida species. Therefore, cultures are preferred to establish an accurate diagnosis, ands they present as white, membranous, villous, wet colonies showing multiple rectangular conidia under the microscope. [19]
For Rhodotorula infections, Gram staining demonstrates a gram-positive yeast with a thin capsule on India ink. Cultures show a coral-pink, moist-to-mucoid yeast-like appearance. They differ from Candida species by producing pink-to-red colonies, owing to its inability to ferment sugars and the lack of pseudohyphae. [16]
Histological findings of hyaline and septate filaments that dichotomize at right angles may resemble those of Aspergillus; therefore, cultures are essential in the diagnosis of Fusarium infection. Microscopic visualization from cultures shows fusoid banana-shaped multicellular macroconidia with foot cells at the base. [20, 17]
Chromogenic culture media such as CHROMagar Candida® can be used to identify these species. Finally, molecular biology can be used to distinguish between G candidum and G capitatum. [19]
Management
Medical care
Pharmacologic treatment includes the following agent classes: azoles, polyenes, echinocandins, and pyrimidines (5-fluorocytosine)
For most of the cases, the treatment of choice is an azole, except for those with advanced and extensive disease for which an intravenous drug such as amphotericin B should be used. Treatment variably continues for 6-12 weeks after culture results are negative.
Antifungal prophylaxis is suggested for immunosuppressed patients.
Azoles such as voriconazole or posaconazole may be required in recalcitrant or invasive infections resistant to other antimycotic agents, especially in immunocompromised patients with unusual mycotic infections. [21] Statins may have some antifungal activity. [22]
Cases of Rhodotorula infection are susceptible to 5-fluorocytosine, moderately susceptible to amphotericin B, miconazole, ketoconazole and itraconazole, but resistant to fluconazole. [16]
A novel triterpenoid antifungal, ibrexafungerp, is being developed. It has shown efficacy against Aspergillus species in vitro and against invasive zygomycosis and aspergillosis in animal models. Currently, ibrexafungerp is approved for vulvovaginal candidiasis in the United States. [23]
Surgical care
In addition to medical therapy, surgical debridement may be required, especially in aspergillosis and zygomycosis (mucormycosis). Zygomycosis used to be almost uniformly fatal and still has a mortality rate approaching 20%; therefore, control of underlying disease is essential if possible, together with systemic amphotericin or azole therapy (eg, fluconazole, itraconazole, or posaconazole) and surgical debridement and, as some suggest, hyperbaric oxygen. [24]
Surgery may be further indicated in cases of mycoses to correct any defects resulting from fungal destruction of the maxilla, orbit, and/or cranial base.
Background
This article discusses the following noncandidal oral mycoses: aspergillosis, cryptococcosis, histoplasmosis, blastomycosis, paracoccidioidomycosis, zygomycosis (mucormycosis), oral geotrichosis, Rhodotorula infection, and fusariosis. Although these noncandidal fungal infections are considerably less common than oral candidiasis, they commonly produce subclinical infection, especially pulmonary infections. Immunocompromised persons are at particular risk from these mycoses, and clinical manifestations of infection by these organisms often suggest impaired immune competence. [25] It is in such people that oral lesions are most likely to manifest. Patients at greatest risk from mycoses include those with leukemia, leukopenia, solid tumors, transplants, or HIV disease. [26, 27, 28] Also at risk are premature infants.
Pathophysiology
Aspergillosis
More than 160 species and variants of Aspergillus organisms have been discovered, although only 10 are pathogenic in humans. Aspergillus fumigatus is the most common pathogen, but Aspergillus flavus, Aspergillus glaucus, Aspergillus nidulans, Aspergillus terreus, Aspergillus repens, Aspergillus parasiticus, and Aspergillus niger are also encountered. A flavus is the most virulent.
Aspergillus species are the most common environmental fungi, being prolific saprophytes in soil and decaying vegetation. Inhalation of the conidia is likely very common, but unless the inhalation is massive or unless the host is immunocompromised, clinical disease is rare. Aspergillosis is found worldwide and its prevalence is increasing. It is now the most prevalent mycosis second only to candidosis.
The organisms exist as prolific saprophytes in soil and decaying vegetation. Inhalation of the organisms allows for their germination and colonization in the mucosa of the respiratory tract, including the mouth. Lesions may be established primarily in the oral mucosa, but they more commonly begin in the mucosa of the maxillary sinus. They may appear in the oral cavity after local invasion and/or destruction of the surrounding structures. Inhalation of the spores is common, although clinical disease is rare unless the individual is immunocompromised by medication (eg, chemotherapy, [28] organ transplantation immunosuppression) or disease (eg, HIV infection, leukemia, lymphoma). Rarely, aspergillosis has followed dental interventions. [7]
Faustino et al presented the case of a 57-year-old female with no known immunocompromise who presented with persistent Aspergillus infection after a dental extraction. The infection developed into a mandibular osteomyelitis. The patient was treated successfully with itraconazole 400 mg PO daily for 3 months. [29]
Blastomycosis
Blastomycosis is a term sometimes used to include a range of granulomatous systemic mycoses, including North American blastomycosis (Gilchrist disease), South American blastomycosis (paracoccidioidomycosis or Almeida disease), coccidioidomycosis, and cryptococcosis. However, the nomenclature is now restricted mainly to the North American and South American forms of blastomycosis, which involve the viscera, lymph nodes, and mucocutaneous tissues.
Blastomyces dermatitidis causes the North American form, whereas Paracoccidioides brasiliensis causes the South American form. As expected, North American blastomycosis is seen predominantly in the Mississippi, Missouri, and Ohio River valleys in the United States and in southern Canada. However, it is also seen in Africa, India, the Middle East, and Australia, and sporadic cases are seen worldwide. Serotype 1 is seen in North America, and serotype 2 is seen in Africa.
B dermatitidis, which is found in soil and spores, may be inhaled to produce respiratory tract and sometimes disseminated disease, for example in diabetes. [30] Serotype 1 is seen in North America, and serotype 2 is seen in Africa. Outdoor workers are particularly affected, but blastomycosis is increasingly recognized in persons with HIV disease.
Coccidioidomycosis
Coccidioidomycosis is seen mainly in arid parts of the Western hemisphere, such as the southwestern United States, Mexico, Central America, and parts of South America. Inhalation of spores of Coccidioides immitis, found in soil, produces subclinical infection in up to 90% of the population in such areas.
Cryptococcosis
Cryptococcosis is seen worldwide in humans and animals and can produce mucocutaneous lesions. [31] Aspiration of basidiospores, mainly capsular serotype A but sometimes serotype D of Cryptococcus neoformans (a ubiquitous yeast found especially in pigeon feces and present in soil), may lead to infection. Two varieties have been described, which are C neoformans var neoformans (synonymous with capsular serotypes A, D, and AD) and the less common C neoformans var gattii (synonymous with capsular serotypes B and C). C neoformans var neoformans is found in excreta from pigeons, canaries, parrots, and budgerigars and in rotting fruit and vegetables. C neoformans var gattii is associated with a particular tree, the Red River gum tree (Eucalyptus camaldulensis).
Note the image below.
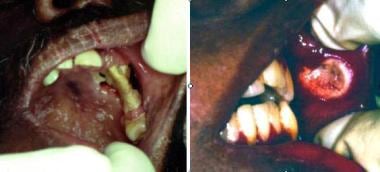 Cryptococcosis. Left image shows solitary, destructive lesion resulting in necrosis of alveolar bone and palatal mucosa; note the superficial pseudomembranous candidiasis of the palate. Right image shows nonspecific chronic ulceration of the buccal mucosa due to cryptococcosis; this is associated with submucosal induration and regional adenopathy. Courtesy of David Sirois, DMD, PhD.
Cryptococcosis. Left image shows solitary, destructive lesion resulting in necrosis of alveolar bone and palatal mucosa; note the superficial pseudomembranous candidiasis of the palate. Right image shows nonspecific chronic ulceration of the buccal mucosa due to cryptococcosis; this is associated with submucosal induration and regional adenopathy. Courtesy of David Sirois, DMD, PhD.
Histoplasmosis
Histoplasmosis is the most frequently diagnosed systemic mycosis in the United States. Sporadic cases are seen worldwide.
Histoplasma capsulatum, the causal organism, is a soil saprophyte found particularly in northeastern and central states such as Missouri, Kentucky, Tennessee, Illinois, Indiana, and Ohio (mainly in the Ohio and Mississippi valleys). The organism has also been found in Latin America, India, the Far East, and Australia. H capsulatum var duboisii is the type mainly found in equatorial Africa.
Histoplasma species are commonly found in bird and bat feces. In endemic areas, the organism is a soil saprophyte, and more than 70% of adults appear to be infected, typically with subclinical manifestations, as a result of inhaling spores.
Histoplasmosis can be an issue in immunocompromised people, such as those with HIV disease, [32] but it can also be seen orally in HIV-infected [33] or occasionally in immunocompetent individuals. [34, 35]
Zygomycosis
Mucor and Rhizopus species are the most common agents to cause zygomycosis. [36] Fungi of the order Mucorales (of the class Zygomycetes) are responsible for most mucormycosis. However, in addition to Mucor and Rhizopus species, organisms from the genera Absidia, Apophysomyces, Mortierella, Saksenaea, Rhizomucor, and Cunninghamella may also be involved. Therefore, the condition is probably better termed zygomycosis.
These fungi are ubiquitous worldwide in soil, manure, and decaying organic matter. Classic zygomycosis occurs worldwide. In some warmer regions, other Zygomycetes such as Conidiobolus coronatus infect a range of animals and can also occasionally cause rhinofacial zygomycosis in humans. Most human cases have been recorded from the Caribbean, Latin America, and Central and West Africa. Sporadic cases are seen worldwide.
Mucoraceae are commonly cultured from the nose, throat, mouth, and feces of many healthy individuals, but infection is rare in otherwise healthy individuals. [37] Infection is typically seen in immunocompromised patients [38] and often may present with palatal perforation. [39]
Note the image below.
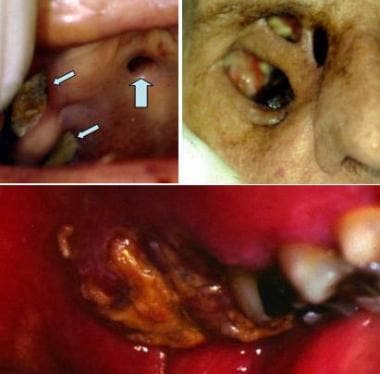 Mucormycosis. Top left image shows multiple, deep ulcerations (arrows) of the hard palate. Top right image shows destruction of the palate and the floor of the orbit (failed skin graft of the right eye after orbital enucleation); this infection originated in the maxillary sinus. Bottom image shows similar deep, destructive ulceration of the left posterior maxillary alveolar bone and mucosa due to mucormycosis of the maxillary sinus. Courtesy of David Sirois, DMD, PhD.
Mucormycosis. Top left image shows multiple, deep ulcerations (arrows) of the hard palate. Top right image shows destruction of the palate and the floor of the orbit (failed skin graft of the right eye after orbital enucleation); this infection originated in the maxillary sinus. Bottom image shows similar deep, destructive ulceration of the left posterior maxillary alveolar bone and mucosa due to mucormycosis of the maxillary sinus. Courtesy of David Sirois, DMD, PhD.
Paracoccidioidomycosis
South American blastomycosis (paracoccidioidomycosis or Almeida disease) is found particularly in Brazil but also in Colombia, Ecuador, Mexico, Venezuela, Uruguay, and Argentina. [40, 41] In Brazil, the disease is endemic in the states of Sao Paulo, Rio de Janeiro, and Minas Gerais. P brasiliensis is responsible and is presumably being inhaled as spores. Subclinical infection is not uncommon in endemic areas. Sporadic cases are seen worldwide. Lesions are often nodular or ulcerative. [42, 43, 44]
Geotrichosis
Geotrichosis is caused by Geotrichum species. In particular, oral lesions may be caused by Geotrichumcandidum. These organisms can be found in various habitats such as plants, soil, milk, cheese, air. and water. Geotrichum species can be found in the microflora of humans, including the oral cavity in many as 30% of healthy individuals. [19, 45, 3] Although respiratory infections are the most frequently reported, oral and cutaneous involvement have been communicated in cases in which immunity is disturbed or severely compromised. [45]
Infections by Saprochaete capitate were formerly grouped as geotrichosis by G capitatum; however, utilizing molecular techniques they have been reclassified since 2004 as members of the Saprochaete genus. [46]
See the images below.
Rhodotorula infection
Rhodotorula is a pigmented yeast in the Cryptococcaceae family. The following species have been mainly described: Rhodotorula glutinis, Rhodotorula minuta, and Rhodotorula mucilaginosa. These organisms may be found in air, soil, lakes, ocean water, and dairy products; they may colonize plants, humans, and other mammals. [20]
Fusariosis
Species of Fusarium are found as plant pathogens causing various diseases on cereal grains. This group of fungi are found with some frequency in invasive fungal infections in immunocompromised individuals. The following species have been most commonly implicated causing infections in humans: Fusarium solani, Fusarium oxysporum, Fusarium verticillioides, and Fusarium moniliforme. [20]
Etiology
Causative fungi include the following:
-
Aspergillosis - A flavus, A terreus, and A fumigatus
-
Cryptococcosis - C neoformans
-
Histoplasmosis - H capsulatum
-
Blastomycosis - B dermatitidis
-
Zygomycosis - Mucor species and Rhizopus species
-
Paracoccidioidomycosis - P brasiliensis
-
Geotrichosis - Geotrichum species
-
Rhodotorula infection - Rhodotorula species
-
Fusariosis - Fusarium species
Epidemiology
Frequency
United States
Because of the ubiquitous presence of these fungi in the environment, exposure is common. However, clinical disease is uncommon except in persons with iatrogenic or pathologic immunosuppression.
International
Because of the ubiquitous presence of these fungi in the environment, exposure is common in endemic areas, and travelers may present with manifestations even years after exposure. However, clinical disease is uncommon except in persons with iatrogenic or pathologic immunosuppression.
Race-, sex-, and age-related information
The deep mycoses can affect individuals of all races; no racial predilection is recognized. The mycoses affect both sexes equally.
The deep mycoses can affect individuals of all ages, although they are more common in adults than in children. Elderly individuals may be at increased risk, although this is often secondary to impaired immunity.
Prognosis
Most fungal infections in apparently healthy individuals are self-limiting or subclinical and have a good prognosis. In a healthy individual, infection is typically self-limited, mainly pulmonary, although latency is commonly established, rather than elimination. Reactivation of latent infection may subsequently occur if the infected individual becomes immunosuppressed.
Primary infection or reactivation in individuals with impaired immune surveillance presents a different scenario in which the disease may continue as a locally invasive and destructive process. Once the organism breaks through local barriers and enters the blood or lymphoreticular system, dissemination is rapid and difficult to control.
If a deep fungal oral lesion develops, the likelihood of self-limiting disease is significantly reduced, and the lesion likely represents a potentially serious underlying infection. Although most deep fungal infections respond to aggressive antifungal therapy, infections can be fatal, particularly mucormycosis and aspergillosis. Deep fungal infections should be viewed as serious and potentially life threatening. Among patients who are immunosuppressed (eg, those with HIV/AIDS, COVID-19, diabetes, leukemia, lymphoma, or iatrogenic immunosuppression as in organ transplantation or cancer therapy), death rates dramatically increase.
Regional destruction of the maxilla by paranasal infections can lead to considerable morbidity, including oroantral fistula with oronasopharyngeal insufficiency and orbital invasion, which may result in loss of the eye.
-
Cryptococcosis. Left image shows solitary, destructive lesion resulting in necrosis of alveolar bone and palatal mucosa; note the superficial pseudomembranous candidiasis of the palate. Right image shows nonspecific chronic ulceration of the buccal mucosa due to cryptococcosis; this is associated with submucosal induration and regional adenopathy. Courtesy of David Sirois, DMD, PhD.
-
Aspergillosis. Note deep mucosal ulceration and nodular expansion of the hard palate. Courtesy of David Sirois, DMD, PhD.
-
Blastomycosis. Top image shows nonspecific papillary nodular lesion on the hard palate. Bottom image shows extensive ulceration involving the skin of the face and neck. Courtesy of David Sirois, DMD, PhD.
-
Histoplasmosis. Top image shows periodontal recession and deep ulceration with exposed necrotic alveolar bone (arrow). Bottom image shows solitary, deep ulceration of the gingivae also associated with necrotic bone (arrow). Courtesy of David Sirois, DMD, PhD.
-
Mucormycosis. Top left image shows multiple, deep ulcerations (arrows) of the hard palate. Top right image shows destruction of the palate and the floor of the orbit (failed skin graft of the right eye after orbital enucleation); this infection originated in the maxillary sinus. Bottom image shows similar deep, destructive ulceration of the left posterior maxillary alveolar bone and mucosa due to mucormycosis of the maxillary sinus. Courtesy of David Sirois, DMD, PhD.
-
Rectangular conidia is observed (cotton blue 400x). Courtesy of Dr. Alexandro Bonifaz.
-
Pseudomembranous form affecting the tongue. Courtesy of Dr. Alexandro Bonifaz.
-
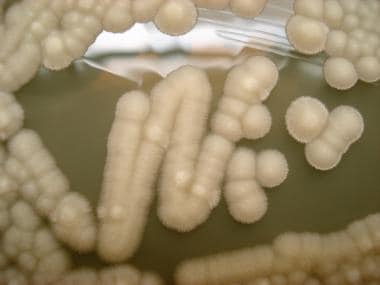
Culture of Geotrichum candidum on dextrose agar. Courtesy of Dr. Alexandro Bonifaz.