Practice Essentials
Onchocerciasis is a common, chronic, multisystemic disease caused by the nematode Onchocerca volvulus. The disease characteristically includes dermatologic (see the image below), lymphatic, ophthalmologic, and systemic manifestations. [1] Human transmission of the disease is caused by a bite from the intermediate host, the black fly (genus Simulium). [2] Black flies breed along waterways, which can vary from small streams to broad rivers. Affected individuals usually live or work within a few kilometers of these sites. Onchocerciasis has long been associated with a high incidence of detrimental effects on socioeconomic development and public health in endemic areas.
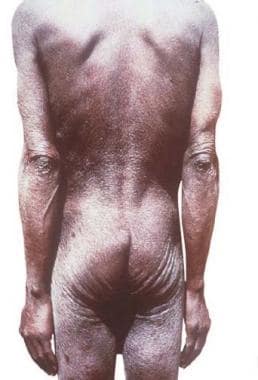 Dermatitis associated with microfilaria. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
Dermatitis associated with microfilaria. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
Related Medscape articles include the following:
Signs and symptoms
Please see Presentation for a full discussion.
The most important task is determining if patients in endemic areas have been exposed to O volvulus via the black fly vector. Obtain a detailed travel history if onchocerciasis is suspected. Travelers to endemic areas may have a particularly severe form of dermatitis.
Other manifestations of onchocerciasis include weight loss, musculoskeletal pain, inguinal hernias, and systemic embolization of microfilariae.
Many patients in endemic regions have associated the disease with secondary amenorrhea, lactation difficulties, spontaneous abortion, infertility, and sterility. However, these associations have never been proven.
Patients are asymptomatic in about 10% of cases.
Diagnostics
Also see Histologic Findings.
The traditional standard diagnosis of onchocerciasis is based on the acquisition of 3- to 5-mg skin snips from an affected area. These snips are immediately immersed in sodium chloride solution and placed under a microscope. The emerging microfilariae are then counted. This method is specific, and it has been the most accurate. However, the use of skin snips is not sensitive for detecting early or mild infections, and this method is becoming increasingly unacceptable to people in endemic communities because of its invasiveness.
The Mazzotti test, now seldom used, involves the administration of 6 mg of diethylcarbamazine (DEC). DEC inhibits neuromuscular transmission in nematodes. Within 2 hours, a positive result produces pruritus and, sometimes, intense inflammation in the areas of dying microfilariae. Other possible effects such as vomiting, conjunctivitis, albuminuria, hypotension, and sudden death (rare) limit its usefulness. Oral DEC was formerly used in treatment of the disease.
The DEC patch test (ie, Mazzotti patch test) involves a topical application of DEC, which produces a local reaction to dying microfilariae at the patch site. [3, 4] This noninvasive test is specific, but it is less sensitive than the skin-snip test. In the future, this test may be more valuable in detecting the recrudescence of infection in onchocerciasis-free zones than in diagnosing the disease.
Enzyme-linked immunosorbent assays (ELISAs), which require only a finger-stick sample, are more sensitive and less invasive than skin-snip tests. This test is used to recognize specific microfilarial antigens. However, ELISA results cannot be used to distinguish past and current infections, a considerable problem in endemic areas.
The polymerase chain reaction (PCR) amplifies repetitive parasite DNA sequences in skin-snip specimens. [5] Compared with skin-snip tests, this method has greater sensitivity in patients with low-level infections. The return of microfilariae to the skin after drug treatment can also be identified earlier with this test. The major disadvantages of PCR are its high cost and invasiveness. A minimally invasive and painless alternative to the use of skin snips is the collection of skin scratches. The superficial layer of the epidermis is removed by carefully scraping the skin with the blunt edge of a disposable lancet. PCR studies of skin scratches, instead of skin snips, have yielded similar results and may be used more often in the future.
Rapid-format antibody card tests are being developed to diagnose onchocerciasis. [6, 7] In these tests, serum samples can be used to detect antibodies, such as immunoglobulin G4 (IgG4) antibodies to recombinant O volvulus antigen Ov16. Tests for additional antibodies are currently under development. Initial studies show good sensitivity and specificity in small numbers of samples and controls using various methods. Some tests can be modified to permit the use of blood samples obtained from less invasive finger sticks. This modification increases the utility of the test for field use. Additional studies are needed to determine the value of these tests in the diagnosis of onchocerciasis.
Microfilariae can often be directly observed by means of slit lamp examination of the cornea and anterior chamber of the eye.
An oncho-C27 antigen detection dipstick assay using urine and tears has been developed. The test can be completed in as short as 3 hours, and the strips maintain reactivity when kept at room temperature for up to 8 months. Sensitivity and specificity were good in an initial study. [8]
Management
Please see Treatment and Medication for a full discussion.
The treatment of onchocerciasis was revolutionized with the introduction of ivermectin in 1987. Ivermectin therapy does not have the adverse reactions of diethylcarbamazine (DEC), and it eliminates the need for 6 weekly injections of suramin. The treatment is suitable for both clinical use and mass distribution in endemic areas. [9, 10, 11]
Ivermectin is a compound derived from the bacterium Streptomyces avermitilis. [12] The drug causes nematode paralysis by impairing neuromuscular function. Ivermectin not only prevents ocular disease but also improves and eliminates the skin disease. A single dose of 150 μg/kg clears the microfilariae from the skin for several months. Ivermectin temporarily decreases the release of microfilariae, but it does not kill adult worms.
Adverse reactions are similar to the responses of the body to dying microfilariae, but the intensity and rate of development are increased. Adverse effects include fever, edema, pruritus, lymphadenitis, and body pains.
The frequency and duration of ivermectin therapy still is being debated. As many as 33% of patients in nonendemic areas are cured with only 1 dose of ivermectin, but most patients require additional therapy. [13]
Resistance to ivermectin may be emerging. A study from Ghana showed that female worms isolated from skin snips in some communities were not responsive or resistant to the antifecundity effects of ivermectin. [14]
Moxidectin is an antiparasitic drug approved by the US Food and Drug Administration (FDA) in June 2018 to treat onchocerciasis in patients aged 12 years or older. The World Health Organization initiated clinical trials for use in onchocerciasis in 2009. Moxidectin is closely related to ivermectin, but it has a more sustained reduction in microfilarial levels. FDA approval was based on a double-blind, parallel group, superiority trial (n=1472) that compared moxidectin (8 mg PO once) with ivermectin (150 mcg/kg PO once). [15] The trial took place in Ghana, Liberia, and the Democratic Republic of the Congo. Results showed skin microfilarial loads (ie, parasite transmission reservoir) were lower from month 1 to month 18 after moxidectin treatment than after ivermectin treatment, with an 86% difference at month 12. Moxidectin would therefore be expected to reduce parasite transmission between treatment rounds more than ivermectin could, thus accelerating progress towards elimination. [15]
Small studies have shown that administration of doxycycline for 6 weeks in addition to ivermectin therapy led to Wolbachia depletion followed by interruption of embryogenesis and reduction in microfilarial loads lasting 18 months. [16]
Strategies targeting eradication of Wolbachia have been undertaken to identify alternative agents for treatment. A study of 5 weeks of doxycycline use without ivermectin showed a reduction in live worms in nodulectomy specimens. [17, 18] Additional studies using azithromycin [19] or rifampicin [20, 21] for Wolbachia eradication have been less efficacious than studies using doxycycline; however, further research may support the use of these agents as alternatives for those intolerant of doxycycline.
Suramin is used less often than ivermectin and given intravenously. However, patients coinfected with O volvulus and Loa loa should not be treated with ivermectin, due to a heightened risk of encephalitis. [1] Accordingly, testing for Loa loa, especially in loiasis-endemic areas, is important.
Pathophysiology
In 1875, John O'Neill first observed O volvulus microfilariae in a case of "craw-craw," as onchocerciasis is known in West Africa. Almost 50 years later, Blacklock discovered the vector to be Simulium in Sierra Leone. The main vector in most of Africa is Simulium damnosum; in Ethiopia, Uganda, Tanzania, and the Democratic Republic of the Congo, Simulium neavei is common. In the Americas, the principal vectors are Simulium metallicum, Simulium ochraceum, and Simulium exiguum. Some vectors bite humans rather exclusively, whereas others are zoophilic to varying degrees. Animal reservoirs of O volvulus have not been found.
When a black fly takes a blood meal from an infected human, they also ingest onchocercal microfilariae in the skin. Surviving microfilariae in the black fly burst through the peritrophic membrane formed by the blood meal, invade the midgut, and advance to the thoracic muscles. The differentiation of these microfilariae into L1 larva begins in muscle within 28 hours after the blood meal. The first molt produces L2 larva within 96 hours, followed by the second molt, which produces L3 larva by day 7. The infective L3 larva migrates to the insect's head and mouth for future deposition into human skin during the next blood meal.
After L3 larvae are transmitted to human skin, those that survive molt within 1 week to form L4 larva. Their development into male and female forms is completed by 1-3 months. The adult worms reside in the deep dermis and fascial planes. Thick, fibrous, subcutaneous nodules called onchocercomas are formed as the result of the development of scar tissue around the adult worms. These onchocercomas average 3 cm in diameter, and they typically contain 2-3 female adults and 1-2 male adults. The nodules typically are surrounded by eosinophils and lymphocytes that are ready to attack the newly produced microfilariae.
Adult worms isolated in nodules are not directly harmful to the patient. Their progeny, which are released from the nodules, are responsible for most of the damage related to onchocerciasis. Nonetheless, to control the disease, the identification and removal of these onchocercomas is critical. Ivermectin is the most effective agent against microfilariae (see Management above). Currently, available agents that are effective against adult worms are toxic. For this reason, surgical removal of the adult worms is important.
Within 10-12 months after the initial infection, adult female worms start producing microfilariae, which have an average lifespan of 6 months to 2 years. The reproductive life of the adult averages 9-11 years. During this time, female worms may release 1300-1900 microfilariae per day. The maximal production of offspring occurs during the first 5 years of the worm's reproductive life, after which this activity declines in a linear fashion.
The microfilariae released from the nodules easily traverse the skin and connective tissue. The subepidermal lymphatics and the anterior chamber of the eye are the most common migration sites. The microfilariae can also be found in the blood, cerebrospinal fluid, urine, and internal organs. More than 100 million microfilariae may be present in severely affected individuals.
A symbiotic relationship has been demonstrated between Wolbachia bacteria and filarial nematodes, including O volvulus. [22] Wolbachia species are essential for nematode fertility. Embryogenesis in the female worm is disrupted when Wolbachia numbers are depleted. In addition, murine experiments suggest that corneal inflammation secondary to onchocerciasis may be caused by endotoxins produced by Wolbachia. Results from several small trials suggest that clearance of Wolbachia with antibiotic therapy affects transmission and can reduce and prevent onchocerciasis-related blindness.
Etiology
In 1875, John O'Neill first observed O volvulus microfilariae in a case of craw-craw, as onchocerciasis is known in West Africa. Almost 50 years later, Blacklock discovered that the vector in Sierra Leone was a Simulium organism. The main vector in most of Africa is S damnosum; in Ethiopia, Uganda, Tanzania, and the Democratic Republic of the Congo, S neavei is common.
The principal vectors in the Americas are S metallicum, S ochraceum, and S exiguum. Some vectors bite humans rather exclusively, whereas others are zoophilic to varying degrees. Animal reservoirs of O volvulus have not been found.
Epidemiology
Frequency
United States
Onchocerciasis is rare in the United States. All reported cases result from the immigration of individuals from endemic areas.
International
Onchocerciasis is a major public health problem in many parts of the world. The disease is endemic in 37 countries in Africa, Latin America, and Yemen. In 1995, an estimated 123 million people were at risk of contracting the disease according to the World Health Organization Expert Committee on Onchocerciasis. Another 17-18 million people were estimated to be infected.
Approximately 95% of all infected people live in Africa. The disease is most severe along the major rivers in 30 countries across the northern and central areas of the continent. Nigeria, Ethiopia, Cameroon, Uganda, and the Democratic Republic of the Congo have the largest number of infected people.
In Latin America, onchocerciasis can be found in Brazil, Venezuela, Colombia, Ecuador, and Guatemala, as well as in the southern mountainous states of Chiapas and Oaxaca in Mexico. [23]
Race-, sex-, and age-related information
To the author's knowledge, no current well-described studies have been performed to determine the frequency of onchocerciasis in specific races.
The disease generally affects more men than women, although sex-related differences may not be apparent until the patient reaches a certain age.
Sex-related differences are more pronounced in high-transmission areas, particularly the savanna.
The overall trend is partially attributed to increased exposures in men, which are related to the occupational risk in farmers, fishermen, and other workers.
The prevalence of onchocerciasis is lowest in individuals aged 0-10 years. Afterward, the prevalence sharply increases, with a peak in individuals aged 20-30 years.
Prognosis
The prognosis for onchocerciasis is good in patients who receive proper therapy before irreversible eye lesions develop. Ivermectin is effective in reducing the skin manifestations of the disease; it thereby reduces morbidity and improves the patient's quality of life.
In 1995, an estimated 270,000 people were blinded and another 500,000 had severe visual impairment as a result of the disease. A multicountry study showed that more than 30% of the population in endemic areas have onchocercal dermatitis. [24] In a survey of skin disease in 7 endemic sites in 5 African countries, 40-50% of adults reported troublesome itching. Blindness is not associated with excess mortality. However, increasing microfilarial load is associated with mortality in both males and females.
-
Leopard-spot pattern of depigmentation on the shins. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
-
Dermatitis associated with microfilaria. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
-
Hanging groin sign. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
-
Hanging groin sign. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
-
Onchocercoma. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
-
Onchocercoma. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
-
Photomicrograph of a skin biopsy specimen from a patient with onchocerciasis. A worm is shown in cross-section. Courtesy of Brooke Army Medical Center teaching file. All images are in the public domain.
-
Photomicrograph from a gravid female worm (hematoxylin and eosin]). Courtesy of Brooke Army Medical Center. All images are in the public domain.






