Practice Essentials
In 1988, Santa Cruz and Aronberg [1] first described targetoid hemosiderotic hemangioma (THH), which is a benign vascular neoplasm that some have postulated may represent a reactive condition. [2] Also termed hobnail hemangioma, targetoid hemosiderotic hemangioma typically presents as a solitary papule affecting the limbs or trunk of young or middle-aged people. Recognition of characteristic features is important to avoid misdiagnosis, as clinically and histopathologically, targetoid hemosiderotic hemangioma can mimic disorders such as melanoma, Kaposi sarcoma (KS), and angiosarcoma. Note the images below. Because the histology of targetoid hemosiderotic hemangioma is reproducible, its clinical features variable, and the hobnail phenomenon not specific, some authors favor the designation of "superficial hemosiderotic lymphovascular malformation" instead of HH or targetoid hemosiderotic hemangioma. [3]
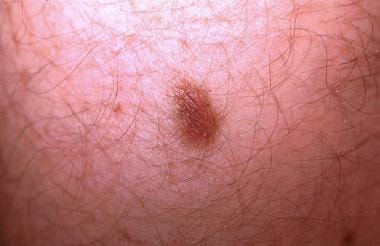 Most targetoid hemosiderotic hemangiomas do not exhibit a targetoid appearance. An older waning lesion is presented showing a 2-toned papule with a dark brown center surrounded by a tan-brown rim. This clinical image can be confused with a melanocytic nevus or dermatofibroma. This 20-year-old patient described episodic changes that varied from a larger violaceous papule surrounded by an erythematous halo (target lesion) to the illustrated lesion.
Most targetoid hemosiderotic hemangiomas do not exhibit a targetoid appearance. An older waning lesion is presented showing a 2-toned papule with a dark brown center surrounded by a tan-brown rim. This clinical image can be confused with a melanocytic nevus or dermatofibroma. This 20-year-old patient described episodic changes that varied from a larger violaceous papule surrounded by an erythematous halo (target lesion) to the illustrated lesion.
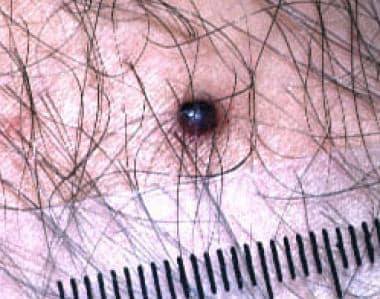 Targetoid hemosiderotic hemangioma mimicking nodular malignant melanoma. Note the dark black papule surrounded by a faint brown rim.
Targetoid hemosiderotic hemangioma mimicking nodular malignant melanoma. Note the dark black papule surrounded by a faint brown rim.
Patient education
Because targetoid hemosiderotic hemangioma can mimic malignant skin tumors, advise patients who have had a targetoid hemosiderotic hemangioma to seek dermatologic consultation should they find another similar lesion on self-examination.
Signs and symptoms
Also see Physical Examination.
Targetoid hemosiderotic hemangioma (THH) (hobnail hemangioma) is a benign vascular tumor that typically manifests as a small, single lesion on the extremity or trunk of a young to middle-aged adult. It has a variable clinical appearance, but most can be described as annular lesions with a central violaceous papule surrounded by an eccentric ecchymotic ring that can exhibit a targetoid appearance. The wide variation in clinical appearance and variegation of color explains why these lesions can be mistaken for a hemangioma, melanocytic nevus, or melanoma.
Lesions generally are asymptomatic, but they may be painful, change color, increase in size, or exhibit cyclical or episodic change. These changes typically are described as enlargement with deepening of color hue, followed by decrease in size and diminished color intensity.
Cases with cyclical morphologic changes have been correlated with hormonal events of the menstrual cycle, although it should be noted that some of these cases have not been documented rigorously from a microscopic standpoint and can represent other processes such as endometriosis.
Spontaneous regression without scarring has been described with recurrence in 1 of 2 cases reported. [4] 13 A separate spontaneous case of regression and recurrence with no identified traumatic trigger has also been described. [5]
Diagnostics
Laboratory testing is not necessary in targetoid hemosiderotic hemangioma (THH) once a firm histopathologic diagnosis has been made. Occasionally, testing for human herpesvirus 8 and the human immunodeficiency virus is relevant, if the clinical and pathologic evaluation of the lesion has raised the issue of Kaposi sarcoma (KS) in the differential diagnosis. [6]
In many cases, dermoscopy can determine if a vascular lesion is present. Dermatoscopic evaluation can identify characteristic red or blue-black lagoons of superficial vascular ectasias, as similarly identified in angiokeratoma and hemangioma. Background shows brownish coloration secondary to hemosiderin deposition, not the pigment network or pigment globules expected in a melanocytic proliferation. The most common dermoscopic pattern in targetoid hemosiderotic hemangioma (71.4% of all cases) is the presence of central red and dark lacunae and a peripheral circular reddish-violaceous homogeneous area. [7]
In a description of 3 THH cases by Biondo et al, the common dermoscopic traits were homogeneous central area, red and dark lacunae, a red-violaceous ring around the periphery, whitish structures, peripheric vascular structures, yellowish intermediate areas, and a peripheral pigment network. [8]
If the clinical diagnosis is questionable or if clinical or patient concern exists regarding possible malignancy, a skin biopsy is warranted for definitive diagnosis. If punch biopsy is used, sampling of the central papular area produces the most diagnostic information. If the lesion is large, an excisional biopsy is helpful in avoiding possible sampling error.
Immunohistochemically, the dilated and slitlike vascular vessels will be labeled by lymphatic endothelial markers D2-40 and VEGFR-3 and will be negative or only focally positive for vascular endothelial markers such as CD34 and CD31, as well as for alpha-smooth muscle actin, which marks surrounding pericytes. [9] In situ hybridization for human herpesvirus 8 (HHV-8), which is found in more than 90% of lesions of KS, is not identified in the endothelial cell nuclei of targetoid hemosiderotic hemangioma. [10]
Also see Histologic Findings.
Management
Medical care is not necessary for targetoid hemosiderotic hemangioma (THH), a benign lesion.
Simple excision is curative and provides for correct microscopic diagnosis. Since targetoid hemosiderotic hemangioma is benign, lesions commonly are removed for diagnostic or cosmetic reasons.
Consultation with a dermatologist can prove helpful in the clinical diagnosis; alternatively, lesions can be biopsied and submitted to a dermatopathology laboratory for microscopic diagnosis.
Pathophysiology
The exact pathogenesis of targetoid hemosiderotic hemangioma (THH) is unknown, but some authorities have postulated trauma to lymphatic vessels with development of lymphatic-vascular microshunts play a key role in the pathogenesis of the defining features of targetoid hemosiderotic hemangioma: dilated vascular spaces with hobnailed endothelial cells, extravasation of red blood cells and hemosiderin deposits, fibrosis, and inflammation. Recent studies have demonstrated that THH vessels are lymphatic in origin. The absence of Wilms tumor 1 antigen expression supports a malformative rather than neoplastic etiology. [11, 12]
One theory states that targetoid hemosiderotic hemangioma is the result of trauma to a preexisting hemangioma that subsequently is altered by thrombosis and recanalization; however, reports fail to document a preexisting vascular tumor, such as lobular capillary hemangioma or congenital vascular malformation, in the 100-plus cases reported to date. However, a 2015 case series by AbuHilal M et al reported three congenital pediatric cases or 3 (50%) of 6. [13]
A second theory suggests that trauma disrupts vasomotor enervation. This leads to an inability to regulate blood flow and results in progressive vascular ectasia, which is characteristic of targetoid hemosiderotic hemangioma. This scenario also has been postulated as a mechanism contributing to the development of acquired port-wine stains. No documented aberration in nerve fiber density in targetoid hemosiderotic hemangioma exists to support this theory.
A third theory proposes that trauma obstructs or destroys draining lymphatics or veins. This leads to proximal compensatory vasodilation and subsequent formation of a hemolymphangioma. This theory is supported by microscopic findings, such as fibrosis and inflammation (scar formation), extravasation of red blood cells and hemosiderin deposition (bruising), telangiectases (secondary to venous bed blockage or destruction), and lymphangiectases (consequence of destruction of lymphatic drainage). Expression of vascular endothelial cell growth factor receptor 3 (VEGFR-3) and D2-40, lymphatic endothelial cell markers, by targetoid hemosiderotic hemangioma endothelial cells confirms the primarily lymphatic origin of targetoid hemosiderotic hemangioma. [14]
These studies examining vessel origin have revealed microshunts between lymphatic channels and small blood vessels that may well explain many of the histologic features of targetoid hemosiderotic hemangioma, such as aneurysmatic microstructures (telangiectases/lymphangiectases) and erythrocytes within histologically apparent lymphatic spaces. The hemolymphatic nature of targetoid hemosiderotic hemangioma would also explain the clinicopathologic overlap with solitary angiokeratoma, an acquired vascular tumor that is also suspected to arise secondary to trauma and is characterized by superficial dermal dilated vessels (telangiectases).
No clear indication exists that targetoid hemosiderotic hemangioma is a true neoplastic process. Incomplete biopsies and complete excisions typically have not been followed by recurrence. To the contrary, reports of waxing and waning lesions suggest a reactive nature. In some cases, this may be the result of the hormonal influence on vasomotor stability that occurs during pregnancy and the menstrual cycle. [15, 16, 6, 17] Spontaneous resolution can be followed by recurrence and is not associated with any scarring. [12, 4, 18]
Etiology
Trauma is the only known predisposing factor for targetoid hemosiderotic hemangioma (THH). [19, 20] Reports of targetoid hemosiderotic hemangioma secondary to irritation from a belt and arthropod assaults exist. Histologic findings of foreign body giant cell reaction in a few cases points to a preceding injury. Episodic changes of enlarging and diminishing hemorrhagic/hemosiderotic halo also implicate recurring trauma or vessel fragility.
Hormones can influence clinical morphology, resulting in the cyclic changes of waxing and waning diameter and peripheral color. Estrogen is believed to mediate pregnancy-related vessel changes, such as spider telangiectases, and estrogen is known to promote vascular permeability and fragility, venous distensibility, increased blood flow, and vasomotor instability. [21] In targetoid hemosiderotic hemangioma, fluctuating estrogen levels may result in vessel instability and leakage. Of note, hormonal receptors have not been identified histologically in lesions of targetoid hemosiderotic hemangioma noted to cyclically change with the menstrual cycle.
Epidemiology
Frequency
United States
Prevalence is unknown. Targetoid hemosiderotic hemangioma (THH) represents less than 0.1% of solitary pigmented lesions presenting to the Albany Medical College pigmented lesion clinic. From a dermatopathologic perspective, the Albany Medical College Dermatopathology service identified 33 cases out of more than 90,000 (0.1%) dermatopathologic accessions over a 3-year period.
International
Global incidence of targetoid hemosiderotic hemangioma is unknown but presumably is similar to that observed in the United States.
Race-, sex-, and age-related information
No known racial associations exist, although THH has been mostly reported in Whites.
Males appear to be affected more frequently by targetoid hemosiderotic hemangioma than females (male-to-female ratio of 1.4:1). Episodically changing targetoid hemosiderotic hemangiomas have been described more frequently in females than in males.
In THH, patient age varies from 5-72 years, and most patients present during their 20s and 30s. An idiopathic case has been reported in a 10-year-old Indian male. [22]
Prognosis
With incisional biopsy or complete excision, the prognosis is excellent, as expected in association with a benign lesion. Reports in the literature clearly indicate that targetoid hemosiderotic hemangioma does not recur, does not invade locally or become locally destructive, and does not spread to other body sites
Rarely, spontaneous resolution can occur, but a risk for recurrence exists. [4, 18]
-
Most targetoid hemosiderotic hemangiomas do not exhibit a targetoid appearance. An older waning lesion is presented showing a 2-toned papule with a dark brown center surrounded by a tan-brown rim. This clinical image can be confused with a melanocytic nevus or dermatofibroma. This 20-year-old patient described episodic changes that varied from a larger violaceous papule surrounded by an erythematous halo (target lesion) to the illustrated lesion.
-
Targetoid hemosiderotic hemangiomas are biphasic vascular tumors that show superficial telangiectases, often lined by hobnailed endothelial cells and deeper slitlike vascular spaces. Note the solitary angiokeratomalike change: the irregular epidermal hyperplasia overlying numerous, blood-filled, dilated vascular spaces in which the lumens diminish with descent into the subjacent dermis (hematoxylin and eosin, original magnification X40).
-
Siderophages and extravasated red blood cells are located at the periphery and between the deep dissecting vascular spaces of targetoid hemosiderotic hemangioma. Prussian blue stain for iron outlines the margins and highlights abundant hemosiderin deposition (Prussian blue, original magnification X100).
-
Targetoid hemosiderotic hemangioma mimicking nodular malignant melanoma. Note the dark black papule surrounded by a faint brown rim.
-
Histologically, targetoid hemosiderotic hemangioma (hobnail hemangioma) can mimic both malignant processes (low-grade angiosarcoma variants) and cutaneous manifestations of systemic disease (Kaposi sarcoma in AIDS). In this instance, the promontory sign of Kaposi sarcoma is replicated by targetoid hemosiderotic hemangioma. Lymphangiectases envelop an arteriole and are surrounded by dilated blood-filled telangiectases (hematoxylin and eosin, original magnification X200).
-
Close-up of a targetoid hemosiderotic hemangioma. Note the ectatic vessels (lagoons) in the central violaceous papule.
-
Solitary angiokeratoma with an erythematous halo (as compared to early targetoid hemosiderotic hemangioma). Histologically, this lesion was found to have deposition of hemosiderin, extravasated red blood cells, lymphangiectases, and focally small slitlike vascular spaces in the reticular dermis similar to that of targetoid hemosiderotic hemangioma.
-
Hobnailed or protuberant endothelial cells characteristic of the superficial vessels of targetoid hemosiderotic hemangiomas. Note the extravasated red blood cells next to the empty and ectatic vascular spaces (hematoxylin and eosin, original magnification X400).
-
Dissecting or pseudoangiosarcomatous vascular spaces of targetoid hemosiderotic hemangioma. Note the absence of marked cytologic atypia and the overlap of endothelial cells that is expected for angiosarcoma (hematoxylin and eosin, original magnification X200).




