Overview
Cryotherapy, also known as cryosurgery, is a commonly used in-office procedure for the treatment of a variety of benign and malignant lesions. In one report, cryotherapy was the second most common in-office procedure after skin excision. The mechanism of destruction in cryotherapy is necrosis, which results from the freezing and thawing of cells. Treated areas reepithelialize. Adverse effects of cryotherapy are usually minor and short-lived.
Dermatologists have used cryotherapy since the turn of the century. After the development of the vacuum flask to store subzero liquid elements, such as nitrogen, oxygen, and hydrogen, the use of cryotherapy dramatically increased. By the 1940s, liquid nitrogen became more readily available, and the most common method of application was by means of a cotton applicator. In 1961, Cooper and Lee [1] introduced a closed-system apparatus to spray liquid nitrogen. In the late 1960s, metal probes became available. By 1990, 87% of dermatologists used cryotherapy in their practice. [2] The general advantages of cryotherapy are its ease of use, its low cost, and its good cosmetic results. Most skin cancers are treated with excision or other destructive procedures, such as electrodesiccation and curettage. Superficial basal cell skin cancers [3] and Bowen disease can be treated with cryotherapy.
Recurrence rates for primary basal cell carcinoma vary with treatment modality. The 5-year recurrence rate for cryotherapy may be as low as 7.5% if lesions are chosen judiciously. [4] This percentage compares favorably with published recurrence rates following other procedures. Published rates include surgical excision, 10.1%; curettage and electrodesiccation, 7.7%; radiation therapy, 8.7%; and all non-Mohs modalities, 8.7%. Because these percentages are derived from various studies, rather than 1 randomized controlled study comparing the different modalities, they should be viewed as rough approximations. Well-circumscribed tumors are most suitable for cryotherapy. The indolent local growth of these well-circumscribed tumors accounts for the high cure rates quoted in the literature.
In most instances, reimbursement for cryotherapy treatment of skin cancers is at the same rate as for destruction of benign lesions.
Mechanism of Action
The mechanism of action in cryotherapy can be divided into 3 phases: (1) heat transfer, (2) cell injury, and (3) inflammation.
Heat transfer
The mechanism by which cryotherapy destroys the targeted cells is the quick transfer of heat from the skin to a heat sink. The most commonly used cryogen is liquid nitrogen, which has a boiling point of -196°C. The rate of heat transfer is dependent on the temperature difference between the skin and the liquid nitrogen.
When using the spray cryotherapy technique, the liquid nitrogen is applied directly on the skin, and evaporation (boiling heat transfer) occurs in which the heat in the skin is quickly transferred to the liquid nitrogen. This process results in the liquid nitrogen evaporating (boiling) almost immediately.
When using a cryoprobe for cryotherapy, conduction heat transfer occurs where the heat is transferred via the copper-metal probe.
Cell injury
Cell injury occurs during the thaw, after the cell is frozen. Because of the hyperosmotic intracellular conditions, ice crystals do not form until -5°C to -10°C. The transformation of water to ice concentrates the extracellular solutes and results in an osmotic gradient across the cell membrane, causing further damage. Rapid freezing and slow thaw maximize tissue damage to epithelial cells and is most suitable for the treatment of malignancies. Fibroblasts produce less collagen after a rapid thaw. [5] Therefore, a rapid thaw may be more suitable for the treatment of keloids or benign lesions in areas prone to scarring. [6]
Freeze damage can be seen when a steak is defrosted from the freezer. The steak juices that are seen when fully thawed represent the intracellular liquid that has escaped because of the damage to the cell wall. Low temperature also ensures maximum damage by further concentrating electrolytes intracellularly.
Keratinocytes need to be frozen to -50°C for optimum destruction. Melanocytes are more delicate and only require a temperature of -5°C for destruction. This fact is the reason for the resulting hypopigmentation following cryotherapy on darker-skinned individuals. Malignant skin cancers usually need a temperature of -50°C, while benign lesions only require a temperature of -20°C to -25°C.
Inflammation
The last response to cryotherapy is inflammation, which is usually observed as erythema and edema. Inflammation is the response to cell death and helps in local cell destruction.
A thorough cryotherapy treatment causes basement membrane separation, which may result in blister formation.
Treatment Modalities Using Cryotherapy
Various methods have been devised in the use of cryotherapy of lesions. They include the spray freeze technique, the applicator technique, the cryoprobe method, and the thermo-coupler method.
Liquid nitrogen is the best and universal freezing source because of its low boiling point and its ease of use. Other sources that are used to freeze, such as Freon, carbon dioxide, liquid helium, and nitrous oxide, do exist, but they are not as efficient in destroying lesions because of their higher boiling points.
Prior to freezing the lesion, the patient must be informed of the procedure, and verbal or written consent must be obtained. A local anesthetic, such as 1% lidocaine, or a topical anesthetic, such as ELA-Max (Ferndale Pharmaceuticals) or EMLA (Astra Pharmaceuticals), can be used. Young children almost always need some type of anesthetic prior to treatment.
Debulking keratotic material with a razor, a No. 11 blade, or a curette by shaving or by curettage facilitates the destruction.
Spray technique
The spray cryotherapy technique is probably the most commonly used method. This method is suitable for most benign and some superficial neoplastic lesions.
Pulsing each spray to avoid an overexpansion of the treatment site prevents complications.
A cone spray technique, as shown in the image below, has been suggested in the past by using an apparatus with 2 open ends; however, such accessories are not readily available and, thus, they are not often used. [7] The cone attachments are round; therefore, they are most suitable for round lesions. For large, irregularly shaped lesions, sequential freezing is necessary.
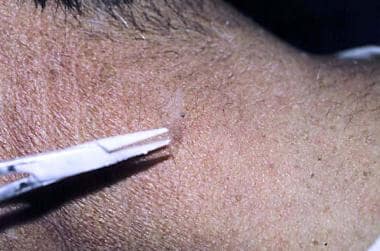
The use of disposable specula instead of nondisposable cone attachments offers several advantages. Disposable specula, as shown in the image below, can be cut with scissors to vary the size and the shape of the aperture. With the advent of HIV and other communicable diseases, the use of a disposable device, such as the plastic specula, is a convenient and simple solution to the problem of contamination. [8]
In the event that a disposable cone attachment is not available, Ashique et al recommend using a disposable paper cup with a window cut for the treatment area or making use of a disposable otoscope speculum. [9]
 To concentrate the freezing and to prevent disease communication (especially in this era of conditions such as HIV disease or hepatitis), disposable cones can be used to concentrate the liquid nitrogen. This disposable otoscope specula can be trimmed to make the aperture larger, or an angle can be cut to make a fusiform shape for oval lesions. Disposable specula come in a variety of sizes, ranging from 2.5-9 mm.
To concentrate the freezing and to prevent disease communication (especially in this era of conditions such as HIV disease or hepatitis), disposable cones can be used to concentrate the liquid nitrogen. This disposable otoscope specula can be trimmed to make the aperture larger, or an angle can be cut to make a fusiform shape for oval lesions. Disposable specula come in a variety of sizes, ranging from 2.5-9 mm.
The nozzle tip of the spray gun is held about 1 cm from the treatment site, and liquid nitrogen is sprayed on the lesion until an ice ball is formed. The operator then palpates the lesion to determine the size of the ice ball created. This process is repeated until an ice ball of the desired size is created. The time for which the lesion is frozen is the freeze time. This freeze-thaw cycle can be repeated, depending on the type of lesion being treated.
Hypopigmentation is a common complication with cryotherapy. This complication is due to the temperature-sensitive melanocytes, which die at the relatively high temperature of -5°C to -10°C. [10] Hypopigmentation complications should be kept in mind, particularly in patients with dark skin tones.
Feathering, the process of gradually and lightly freezing the area surrounding the ice ball to prevent an abrupt edge, can provide better cosmetic results.
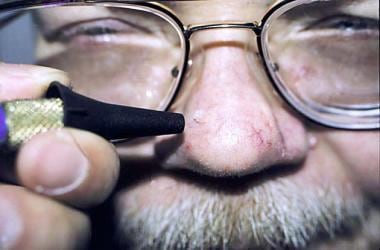
A spray gun is shown in the image below:
 The most common applicator device is the spray gun. This vacuum bottle holds liquid nitrogen and releases the liquid with reasonable accuracy.
The most common applicator device is the spray gun. This vacuum bottle holds liquid nitrogen and releases the liquid with reasonable accuracy.
SARS-CoV-2 precautions [11]
Concern has been expressed over potential coronavirus disease 2019 (COVID-19) exposure to healthcare providers during cryotherapy procedures. As SARS-CoV-2 virus particles have been identified in the feces of infected patients, the British Association for Sexual Health and HIV (BASSH) published a statement in July 2020 asserting that cryotherapy of anogenital warts is not an aerosol‐generating procedure. As such, BASSH maintained that anogenital wart cryotherapy posed no special COVID-19 risk.
However, Searle et al point out that dermatologists often carry out cryotherapy to perioral and perinasal skin and mucosae, which are high-risk sites. Therefore, the authors recommend the use of extensive personal protective equipment and air purifiers/smoke extractors when treating these areas with cryotherapy.
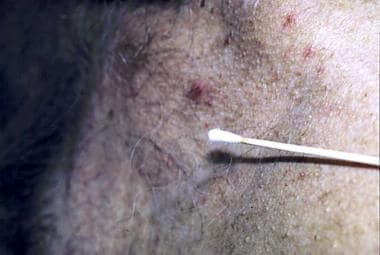
Dipstick applicator method [12]
The dipstick applicator method, as shown in the image below, is the original method used to apply liquid nitrogen to lesions. A cotton-tipped applicator is dipped into liquid nitrogen from a polystyrene cup. The dipstick applicator is then firmly pressed against the lesion for the desired duration. Low temperatures are not achieved in the dipstick applicator method as they are in the spray technique; therefore, this method is suitable only for benign lesions.
Adenovirus is capable of survival in liquid nitrogen; therefore, the same source of liquid nitrogen should not be used with different patients.
Previously unused liquid nitrogen can be placed in a polystyrene cup and then promptly discarded after use for a single patient.
Cryoprobe
Manufacturers have devised various metal attachments to serve as heat-conducting probes for cryotherapy. Copper, because of its high conductivity, is typically used.
A thin film of white petrolatum can be applied to the treatment site, and the cold probe is firmly pressed against the lesion. Many practitioners do not use the probe because it is cumbersome and time consuming.
Lim et al reported good clinical outcome with probe-delivered cryotherapy in patients with primary malignant bone tumors. [13]
Thermo-couple device
To treat malignant lesions, a temperature probe coupled to a digital thermometer that can read to -75°C can be used.
Local anesthetic is injected into the lesion, and a temperature probe is inserted into the estimated depth of the lesion. Usually, a metal or styrene cone is used to concentrate the freeze. The liquid nitrogen is sprayed into the cone until the desired temperature is reached, usually -50°C to -60°C. This process can be repeated until the desired destruction is achieved.
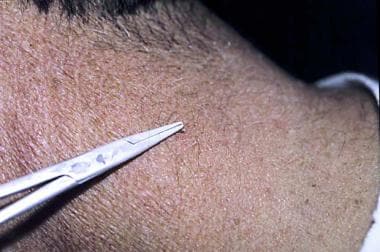
Forceps technique
A forceps technique, as shown below, can also be used. [14]
Clinical Applications
Various lesions have been treated with liquid nitrogen. Because of the histologic differences in lesions, different freeze-thaw cycles are used to successfully destroy them. For most of these lesions, cryotherapy is not the only and often not the best modality for treatment. However, it does represent a valuable alternative for selected patients. For some lesions, such as actinic keratoses, warts, and seborrheic keratoses, cryotherapy is the standard first-line therapy. The time estimates given below must be adjusted depending on the skin temperature, local circulation, skin pigment, and method of delivery. Overfreezing can produce permanent hypopigmentation and scarring.
Pigmented lesions
For melasma, a light, uniform freeze with feathering completed every 4-6 weeks can produce a good cosmetic result in some patients. Other modalities, such as topical depigmenting agents, sunscreens, and chemical peels, are more commonly used to treat melasma.
For tattoos, two 30-second freeze-thaw cycles with 1-mm margins performed every 4-6 weeks can improve some lesions. Newer modalities, especially laser therapy, are more suitable for most tattoos.
Idiopathic guttate hypomelanosis can be treated with one 5-second freeze with feathering performed every 4-6 weeks.
Lentigo can be treated with one 5- to 10-second freeze with feathering completed every 4-6 weeks until resolved. Often, one treatment is all that is required.
Cryotherapy for melanocytic nevi generally is not recommended because these lesions should undergo histologic evaluation. Furthermore, atypical histologic features have been described in benign melanocytic lesions following cryotherapy. These atypical features include loss of maturation and increased staining with HMB-45, which could make the differentiation of benign from malignant melanocytic lesions difficult. [15]
Vascular lesions
AIDS-related Kaposi sarcoma can be treated by performing two 30-second freeze-thaw cycles with 3-mm margins every 4 weeks.
A venous lake can be treated with one 10-second freeze-thaw cycle with a 1-mm margin; usually, 1 cycle is all that is required.
Cherry angiomas can be treated with one 10-second freeze-thaw cycle with a 1-mm margin.
Capillary hemangioma can be treated with two 30-second freeze-thaw cycles with a 1-mm margin.
Vascular malformations of blue rubber bleb nevus syndrome have been treated with a single 10-second freeze. [16]
Cysts and tumors
One 10-second freeze-thaw cycle with a 1-mm margin can be used to treat acne cysts. This method is usually adequate to cause exfoliation and to open pores.
For milia, a short freeze-thaw cycle of 5-10 seconds is usually adequate.
Skin tags can be treated with one 10-second freeze-thaw cycle.
Seborrheic keratosis can be treated with one 10-second freeze-thaw cycle.
Other lesions
One 15- to 30-second freeze-thaw cycle with a 1-mm margin can be used to treat keloids. [17] Cryotherapy of keloids can be painful. Other modalities, such as intralesional corticosteroid injection, are more commonly used.
Rhinophyma can be treated with two 30-second freeze-thaw cycles of the entire nose at 4-week intervals. [18]
Most nonmelanoma skin cancers, such as squamous cell carcinomas and basal cell carcinomas (BCC), should be treated by excision with sufficient margins, by Mohs surgery, or with radiation. When cryotherapy is used in the treatment of BCC lesions, the lesions should be frozen to a temperature between -60°C and -50°C. One study showed that a double freeze-thaw cycle of 30 seconds each was optimal for BCC lesions of the face, while a single 30-second freeze-thaw cycle was more suited for BCC lesions on the trunk. [19]
The freeze time for actinic keratoses is highly variable, depending on skin temperature and the thickness of the lesion. The combination of field-directed therapy and cryotherapy reportedly is more effective than cryotherapy alone. [20, 21]
Warts can usually be treated with two 15- to 30-second freeze-thaw cycles, depending on the thickness; however, some suggest that duct tape may be a more cost-effective and equally effective removal method. [22] Clebak et al suggest using a "cryoblast" technique for the treatment of plantar warts. This approach involves removing the spray tip from the cryosurgery canister and spraying in brief, 1-2 second bursts until an ice ball of the desired size is formed. [23]
In a study of 74 children with molluscum contagiosum, imiquimod cream 5% was compared with cryotherapy. Cryotherapy was more rapid but was associated with more significant adverse effects (bullae formation, pigmentary changes, pain, superficial scarring). The effects of imiquimod treatment were of slower-onset but the treatment was virtually free of adverse effects. The authors suggest that in children, imiquimod may be better for numerous small lesions and cryotherapy may be better for large, solitary lesions. [24]
Cryotherapy reportedly is an effective treatment for epidermal nevi. Success was achieved with 2 cycles using an open-spray technique at 10-15 seconds each. Ten patients had successful results after 2-5 sessions, with no scarring. One patient had a relapse after 8 months, and 1 patient developed hypochromic scarring that resolved after 6 months. [25]
Lobomycosis is typically treated with surgical excision; however, in patients with multifocal lesions, treatment with cryosurgery and long-term itraconazole has been used with success. In one study, 5 sessions of two 2-minute freeze cycles accompanied by oral itraconazole for 6 months led to complete resolution, sparing a surgical procedure. [26]
Cryolipolysis
A novel use of cryotherapy is selective cryolipolysis. This is when fat cells are subjected to near-subzero temperatures via surface cooling to cause selective cell death of adipose cells without harming the overlaying skin or nerves and vasculature within the target area. It was first described in 2008 and was written about in a human population in 2009. [27, 28]
While evidence of achieving a decreased fat layer is suggested by changes in thickness via ultrasound measurements, the long-term results are not yet not fully elucidated; this technology received FDA approval in 2010 (ZELTIQ, CoolSculpting; Pleasanton, Calif). However, the latest evidence indicates a robust patient response (86% saw improvement) with a limited adverse effect profile. Typically, only minimal discomfort occurred. Expected adverse effects are temporary erythema, bruising, and transient numbness, which usually resolve within 14 days of treatment. The most common serious adverse effect is late-onset pain (in 0.1%), occurring 2 weeks after the procedure. This seems to resolve without intervention. [29]
Complications
As with any procedure, complications can occur. Complications can be divided into (1) acute, (2) delayed, (3) prolonged-temporary, and (4) permanent.
Acute complications include headache, pain, and blister formation.
Delayed complications include hemorrhage, infection, and excessive granulation tissue formation.
Prolonged-temporary complications include milia, hyperpigmentation, and change in sensation.
Permanent complications include alopecia, atrophy, keloids, scarring, hypopigmentation, and ectropion formation.
Contraindications
Contraindications can be divided into 2 groups: relative contraindications and absolute contraindications.
Relative contraindications include cold intolerance, cold urticaria, cryoglobulinemia, history of pyoderma gangrenosum, and Raynaud disease.
Absolute contraindications include the use of cryotherapy near the eye margins.
Conclusion
Cryotherapy is a safe and easy to use treatment to destroy many benign and malignant lesions.
The cryotherapy operator must be aware of the complications associated with this modality; for example, a complication is hypopigmentation (dark-skinned individuals are prone to hypopigmentation).
One needs to be aware of the fact that lesions may need to be treated multiple times before resolution.
-
The most common applicator device is the spray gun. This vacuum bottle holds liquid nitrogen and releases the liquid with reasonable accuracy.
-
A cotton-tipped applicator is another method used to treat benign lesions.
-
To concentrate the freezing and to prevent disease communication (especially in this era of conditions such as HIV disease or hepatitis), disposable cones can be used to concentrate the liquid nitrogen. This disposable otoscope specula can be trimmed to make the aperture larger, or an angle can be cut to make a fusiform shape for oval lesions. Disposable specula come in a variety of sizes, ranging from 2.5-9 mm.
-
Photo taken prior to spraying of the lesion by using the cone technique.
-
Forceps or clamps can be used to concentrate the freeze and to prevent collateral damage.
-
A clamp is being used during the freezing treatment shown below. Usually, 2 freeze applications of 15 seconds are required.