Overview
Dermatologic manifestations of renal disease are not uncommon findings in patients with end-stage renal disease (ESRD). Cutaneous examination of patients with ESRD has shown that 50-100% of patients have at least 1 dermatologic condition. A high prevalence of cutaneous disorders is expected, because most patients with ESRD have an underlying disease process with cutaneous manifestations. In addition, uremia and conditions associated with renal replacement therapy are fraught with numerous and, often, relatively unique cutaneous disorders. Consequently, dermatologic manifestations of renal disease may be divided into 3 general categories including: (1) dermatologic manifestations of diseases associated with the development of ESRD, (2) dermatologic manifestations of uremia, and (3) dermatologic disorders associated with renal transplantation.
The image below illustrates several uremia-related cutaneous disorders.
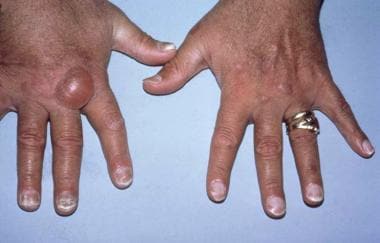 Hands of a transfusion-dependent patient on long-term hemodialysis. Several uremia-related cutaneous disorders are visible. The pigmentary alteration results from retained urochromes and hemosiderin deposition. The large bullae are consistent with either porphyria cutanea tarda or the bullous disease of dialysis. All nails show the distal brown-red and proximal white coloring of half-and-half nails.
Hands of a transfusion-dependent patient on long-term hemodialysis. Several uremia-related cutaneous disorders are visible. The pigmentary alteration results from retained urochromes and hemosiderin deposition. The large bullae are consistent with either porphyria cutanea tarda or the bullous disease of dialysis. All nails show the distal brown-red and proximal white coloring of half-and-half nails.
This article does not discuss systemic disorders, because most of them are discussed in other articles in Medscape Reference. The purpose of this article is to integrate renal and cutaneous aspects of disease as well as highlight some important, although frequently underappreciated, clinical or laboratory findings that ally renal and skin diseases. Recognition of the details may provide clinicians with greater insight into the management of patients.
For patient education information, see Diabetes Center, Cholesterol Center, and Chronic Kidney Disease.
See also Chronic Kidney Disease and Chronic Renal Failure.
Dermatologic Manifestations of Diseases Associated With ESRD
Many cutaneous disorders experienced by patients undergoing dialysis have little to do with the uremic syndrome and are related to the same underlying pathologic process that caused the renal disease. Because dialysis and transplant centers are required to report specific information regarding each patient diagnosed with end-stage renal disease (ESRD) to the United States Renal Data System (USRDS), data regarding the causes of ESRD are readily available in the Annual Data Report published by the USRDS.
Review of the 2019 report reveals that diabetes mellitus remains the most common cause of ESRD, responsible for approximately 42% of all patients on renal replacement therapy. [1] Hypertension accounts for approximately 26% of cases, and glomerulonephritis and cystic kidney diseases account for about 16%, although glomerulonephritis is not as prevalent as it was in the past. [1] The remaining causes of ESRD included vasculitis from an infectious or rheumatologic disease, interstitial nephritis, tumors, cholesterol emboli, and systemic amyloidosis. Infectious causes of glomerulonephritis included streptococcal infections, human immunodeficiency virus (HIV) infection, and hepatitis viral infections, both hepatitis C (HCV) and hepatitis B (HBV). [1]
Systemic lupus erythematosus (SLE) has been the most commonly reported rheumatologic cause of ESRD. Polyarteritis nodosa, granulomatosis with polyangiitis, Henoch-Schönlein purpura, scleroderma, and otherwise nonspecified vasculitides also were reported to have caused ESRD during this period. These systemic disorders and the associated renal diseases and cutaneous manifestations are tabulated in Table 1, below.
Table 1. Dermatologic Manifestations of Diseases Associated with the Development of ESRD (Open Table in a new window)
Systemic Disorder |
Renal Disorder |
Dermatologic Manifestations |
Diabetes mellitus |
Diabetic nephropathy |
Diabetic dermopathy Necrobiosis lipoidica Acanthosis nigricans Eruptive xanthomas Kyrle disease Scleredema |
Systemic lupus erythematosus |
Glomerulonephritis Nephrotic syndrome |
Purpura Chronic cutaneous lupus Subacute cutaneous lupus Photosensitivity Mucosal ulcers Vasculitis Tumid lupus Systemic lupus erythematosus–associated neutrophilic dermatosis |
Henoch-Schönlein purpura |
Glomerulonephritis Vasculitis |
Purpura |
Granulomatosis with polyangiitis |
Glomerulonephritis Vasculitis |
Purpura Subcutaneous nodules Ulcers |
Polyarteritis nodosa |
Glomerulonephritis Vasculitis |
Purpura Subcutaneous nodules Ulcers |
Subacute bacterial endocarditis |
Renal emboli Glomerulonephritis |
Petechiae Purpura |
Cholesterol emboli |
Renal emboli |
Petechiae Livedo reticularis Blue toes |
Hepatitis C virus |
Glomerulonephritis |
Purpura Porphyria cutanea tarda Lichen planus Sclerodermatous plaques Cutaneous polyarteritis nodosa Necrolytic acral erythema |
Human immunodeficiency virus (HIV) |
HIV-associated nephropathy |
Eosinophilic folliculitis Oral hairy leukoplakia Bacillary angiomatosis Kaposi sarcoma |
Systemic sclerosis |
Malignant hypertension |
Diffuse scleroderma |
Amyloidosis |
Nephrotic syndrome |
Purpura Macroglossia |
Fabry disease |
Nephrotic syndrome |
Angiokeratomas |
Nail-patella syndrome |
Renal tubular defects |
Absent/displaced patella Absent/pitted nails |
Tuberous sclerosis |
Renal hamartomas Renal cell carcinoma |
Adenoma sebaceum Ash-leaf macule Periungual fibromas Shagreen patch |
ESRD = end-stage renal disease |
||
Diabetes mellitus
Many cutaneous disorders are associated with diabetes mellitus, [2, 3, 4] including necrobiosis lipoidica diabeticorum, eruptive xanthomas, and diabetic dermopathy. Other characteristic dermatologic manifestations include scleredema, acanthosis nigricans, Kyrle disease (see the following image), [5] and cutaneous changes related to pruritus. One study suggested that of these cutaneous findings, diabetic dermopathy correlates best with internal involvement, including retinopathy, neuropathy, and nephropathy.
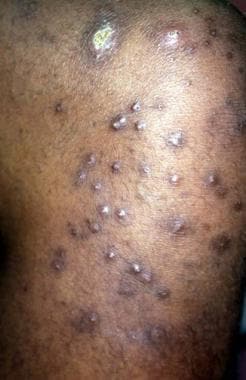 Patients with uremia may develop an acquired perforating disorder. These highly pruritic, hyperkeratotic papules of Kyrle disease are present on the lower extremity of a patient with diabetes and end-stage renal disease.
Patients with uremia may develop an acquired perforating disorder. These highly pruritic, hyperkeratotic papules of Kyrle disease are present on the lower extremity of a patient with diabetes and end-stage renal disease.
Lupus erythematosus
Lupus erythematosus may be classified into 3 subsets including:
-
Chronic cutaneous lupus erythematosus (CCLE)
-
Subacute cutaneous lupus erythematosus (SCLE)
-
Systemic lupus erythematosus (SLE)
CCLE may be a devastating and disfiguring cutaneous disease, but significant systemic involvement occurs in less than 5-10% of patients. A higher percentage of patients with SCLE have systemic involvement, but severe renal disease is uncommon. [6] Mucocutaneous abnormalities occur in 85% of patients with SLE and may involve the skin, hair, or mucous membranes. [7] Renal disease occurs in 50-100% of patients with SLE and ranges from mildly abnormal findings in urinalysis to rapidly progressive and fulminant renal failure. Anti–double-stranded DNA (dsDNA) is a laboratory marker for patients with SLE at increased risk of developing renal disease. In contrast, patients with SCLE without renal disease more frequently have detectable anti-Ro/SSA and anti-La/SSB antibodies. [8]
Granulomatosis with polyangiitis
Granulomatosis with polyangiitis is an uncommon systemic disorder characterized by granulomatous and necrotizing inflammation of the upper and lower respiratory tracts and the kidneys. Chronic renal failure occurs in 35% of patients. Skin involvement occurs in 14-77% of patients and is associated with a higher frequency of renal involvement. [9, 10] Cutaneous lesions include purpura (most commonly on lower legs), subcutaneous nodules, and ulcers.
Although cutaneous biopsy findings are frequently nonspecific, 2 distinct histologic findings have been described, including leukocytoclastic vasculitis and granulomatous inflammation. Leukocytoclastic vasculitis is more common and is associated with palpable purpura, a more aggressive course, a higher frequency of renal disease, and an overall poorer prognosis.
Polyarteritis nodosa
Diseases that fall under the classification of polyarteritis/periarteritis nodosa (PAN) probably represent a group of distinct disorders with common cutaneous manifestations. These disorders may be subclassified as either systemic PAN or solely cutaneous PAN. Cutaneous lesions are reported to occur in 25-50% of patients with systemic PAN and include livedo reticularis and ulceration due to a necrotizing vasculitis and subcutaneous nodules caused by aneurysms of superficial vessels.
Renal involvement occurs in 25-60% of patients with systemic PAN and is a poor prognostic indicator. Historically, up to 50% of cases of systemic PAN were associated with hepatitis B (HBV) infection; data suggest the rate has fallen to closer to 15%. [11] Alternatively, as many as 14% of cases with cutaneous PAN have been associated with hepatitis C (HCV) infection. [12, 13]
Cholesterol emboli
Cholesterol emboli may affect any organ; however, the skin and kidneys are affected most commonly. The subacute nature of this disorder frequently results in an inaccurate diagnosis; however, skin biopsy may readily reveal the diagnosis. [14] Emboli can develop spontaneously or follow an invasive vascular procedure such as an arteriogram. Embolization may occur either shortly after the procedure or many weeks later. Renal failure resulting from cholesterol emboli may evolve slowly or transpire precipitously. The cutaneous signs of cholesterol emboli include livedo reticularis, petechiae, purpura, and blue toes. Because aortic atherosclerotic plaques are the primary source of emboli, the lower extremities are affected most commonly.
Patients frequently develop constitutional symptoms (eg, fever, myalgias) that may complicate the clinical picture further. Laboratory studies, although not diagnostic, are fairly characteristic, demonstrating not only a leukocytosis and elevated erythrocyte sedimentation rate (ESR) but also an eosinophilia. Specific organ involvement may be reflected in laboratory abnormalities. Serum amylase, creatine kinase, or hepatic transaminases may be elevated in association with pancreatic, muscle, or hepatic involvement, respectively.
Hepatitis C virus
An estimated 3.2 million Americans are infected with hepatitis C virus (HCV), and without treatment the majority will develop chronic liver disease. HCV is also the most common cause of essential mixed cryoglobulinemia. Of patients with HCV, 50% have measurable cryoglobulins, although only one third of these patients are symptomatic.
The most common cutaneous manifestation of cryoglobulinemia is palpable purpura resulting from an immune complex–mediated leukocytoclastic vasculitis. Other cutaneous manifestations of HCV infection include porphyria cutanea tarda (PCT), [15] lichen planus, necrolytic acral erythema (NAE), and cutaneous changes associated with chronic pruritus. NAE is a rare cutaneous condition affecting predominately the hands and feet. Previously considered pathognomonic for HCV infection, NAE has since been found in other conditions. Curiously, zinc deficiency may also be present, although zinc replacement does not always result in improvement in NAE.
Untreated renal involvement occurs in approximately 50% of patients with HCV and may progress to ESRD in approximately 10%. [16] Renal disease, primarily membranoproliferative or membranous glomerulonephritis, results from glomerular damage from circulating immune complexes.
Human immunodeficiency virus
Cutaneous disease occurs in 60-100% of patients infected with human immunodeficiency virus (HIV). Seborrheic dermatitis, the most common cutaneous condition seen in individuals infected with HIV, usually develops early and increases in severity as the CD4 count falls. Other cutaneous disorders are relatively unique to patients with acquired immunodeficiency syndrome (AIDS) and appear only when the CD4 count is critically low, usually less than 200 cells per µL. Included in these disorders are eosinophilic folliculitis, oral hairy leukoplakia, bacillary angiomatosis, and Kaposi sarcoma. [17]
HIV-associated nephropathy (HIVAN) is a syndrome of massive proteinuria, hematuria, and azotemia, most commonly in a normotensive, young black male. [18] The renal histology is that of focal segmental glomerulosclerosis. Data now suggest a direct role for HIV infection and viral replication within renal cells in the pathogenesis of HIVAN. Additional information suggests that early initiation of highly active antiretroviral therapy (HAART) [19, 20, 21, 22] and/or an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-receptor blocker (ARB) [23, 24] may stabilize, or perhaps even restore, renal function in those with HIVAN.
Systemic sclerosis
Systemic sclerosis (SSc) is a multisystem disorder that may affect the skin and several internal organs, specifically the gastrointestinal (GI) tract, lungs, heart, and kidneys. [25] The pathogenesis of SSc is unknown, but the end result is excessive fibrosis. SSc may be divided into 2 discrete types according to the extent of cutaneous involvement and includes limited cutaneous SSc and diffuse cutaneous SSc.
In individuals with limited cutaneous SSc, the onset of sclerosis is gradual and confined to areas distal to the elbows and knees, as well as to the face and neck. Digital ulcers are common and typically follow a long history of Raynaud phenomenon. Esophageal dysmotility may develop in some patients as a part of CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasias).
The prognosis of limited cutaneous SSc is generally good, although pulmonary fibrosis or pulmonary hypertension may develop. Diffuse cutaneous SSc, unlike limited cutaneous SSc, develops acutely and is associated with constitutional symptoms and arthralgias. Cutaneous SSc involves the trunk and areas distal to and proximal to the elbows and knees. Diffuse cutaneous SSc has a worse prognosis than limited cutaneous SSc because of a higher incidence of internal organ involvement, including the kidneys. Other skin changes observed in both forms of SSc include hypopigmentation and hyperpigmentation, xerosis, alopecia, telangiectasias, and pruritus.
Renal involvement occurs in fewer than 20% of patients with SSc and tends to occur early, usually within the first 2 years of the disease and almost always within the first 5 years. Renal disease develops precipitously and without warning, although in some patients, rapidly progressive skin thickening may precede renal disease. Fulminant renal failure is usually associated with malignant hypertension.
Renal involvement is a poor prognostic indicator and, until recently, resulted in either ESRD or death. Immediate hospitalization and use of an angiotensin-converting enzyme inhibitor (ACEI) has improved overall renal outcome greatly.
Amyloidosis
Amyloidosis results from the production and extracellular deposition of an abnormal fibrous protein that has specific characteristics. Several amyloid proteins have been reported to develop in response to pathologic processes. Amyloidosis may be a systemic condition or a strictly cutaneous condition.
Systemic amyloidosis has been associated with lymphoproliferative disorders, as well as several chronic inflammatory conditions including osteomyelitis, rheumatoid arthritis, and tuberculosis. The kidney is a frequent site of amyloid deposition, although any organ may be involved. [26]
Renal manifestation of amyloidosis consists of nephrotic range proteinuria with progressive renal failure. Cutaneous manifestations include macroglossia and pinch purpura. Interestingly, cases of systemic amyloidosis with renal involvement have been reported to occur in the setting of a primary, chronic, and inflammatory cutaneous process, such as acne conglobata, hidradenitis suppurativa, and psoriasis. A routine urinalysis for proteinuria serves as an adequate screen for renal amyloidosis.
Amyloidosis, due to deposition of beta2-microglobulin, can also develop in the setting of dialysis. Although this is a major cause of conditions such as carpal tunnel syndrome and bone cysts, only rarely does cutaneous involvement occur.
Dermatologic Manifestations of Uremia
The lives of many patients with end-stage renal disease (ESRD) are maintained by either hemodialysis or peritoneal dialysis. Unfortunately, dialysis cannot compensate completely for the changes associated with kidney dysfunction. Renal replacement modalities are not as efficient in removing many of the substances readily cleared by a healthy kidney. Nor can dialytic therapy replace the endocrine function lost with renal failure. Most patients with ESRD develop significant metabolic abnormalities, which are related directly to the loss of kidney function.
Acquired abnormalities are numerous and include the development of a metabolic acidosis and anemia, as well as an alteration in calcium-phosphate homeostasis, hyperparathyroidism, hyperlipidemia, and glucose intolerance. These metabolic changes predispose patients with ESRD to bone disease, vascular calcification, and an increase in cardiovascular morbidity and mortality.
In the late 1980s, recombinant erythropoietin became available and has helped alleviate the anemia associated with uremia. Before widespread use of recombinant erythropoietin, many patients required frequent transfusions to maintain an adequate hematocrit. Individuals who required multiple transfusions were at high risk for developing iron overload, as well as hepatitis C (HCV) infection. The amalgamation of these numerous and hazardous metabolic abnormalities is responsible for most of the clinical characteristics of the uremic condition.
Many of the dermatologic manifestations associated with uremia, such as pruritus and xerosis, are prevalent in the ESRD population but are not specific for uremia.
Other manifestations, especially those related to the dialysis procedure, are unique to this population but are not discussed in this article. (See Calcinosis Cutis, Calciphylaxis, Kyrle Disease, Bullous Disease of Dialysis, Nephrogenic Fibrosing Dermopathy.)
This section discusses the other primary dermatologic manifestations associated with uremia including the following:
-
Xerosis
-
Pruritus
-
Pigmentary alteration
-
Half-and-half nails
-
Alopecia
-
Uremic frost
-
Porphyria cutanea tarda
-
Arterial steal syndrome
Xerosis
Significant xerosis occurs for unknown reasons in 50-92% of the dialysis population. Some patients may develop acquired ichthyosis. Some authors have suggested that end-stage renal disease (ESRD)–associated xerosis may be a result of a decrease in water content in the epidermis. Clinical and histologic evaluations have shown an overall decrease in sweat volume in patients with uremia, as well as atrophy of sebaceous glands. These changes can allow dehydration of the stratum corneum; however, when directly measured, the water content of the epidermis in patients with ESRD has not been shown to be decreased.
Because many of the cutaneous changes are similar to those induced by retinoids, hypervitaminosis A also has been implicated as an etiologic culprit. Hypervitaminosis A is common in the dialysis patient; however, the epidermal vitamin A content in patients who have ESRD with xerosis is no different from in patients without xerosis. To date, the exact cause of xerosis in ESRD remains unknown. Perhaps the perceived roughness results from a uremia-induced alteration in corneocyte maturation. Many patients respond to routine use of emollients.
Pruritus
Uremia is the most common metabolic cause of pruritus. Significant pruritus affects 15-49% of patients with chronic renal failure and up to 85% of the dialysis population. However, its incidence in the dialysis population appears to have declined over the past few decades. [27] For some patients, pruritus may be relieved with the initiation of dialysis; however, pruritus more commonly begins approximately 6 months after initiation of dialysis and typically increases with the length of time on dialysis. Development of pruritus has no consistent association with age, sex, race, or precipitating disease. Pruritus may be episodic or constant, localized or generalized, and mild or severe. When localized, the forearms and upper back predominately are affected (see the image below).
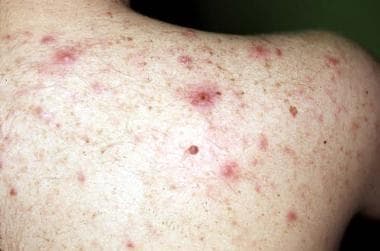
 Chronic scratching resulting from uremic pruritus may result in numerous cutaneous lesions. This patient has many atrophic hyperpigmented scars and an excoriated prurigo nodule on the shoulder.
Chronic scratching resulting from uremic pruritus may result in numerous cutaneous lesions. This patient has many atrophic hyperpigmented scars and an excoriated prurigo nodule on the shoulder.
Pruritus frequently affects the patient's sleep pattern and psychologic well-being. Cutaneous manifestations of pruritus include excoriations, prurigo nodularis, and lichen simplex chronicus. Mechanisms of uremia-induced pruritus are poorly understood and are believed to result from metabolic disequilibrium. Because pruritus is not seen with acute renal failure, changes in blood urea nitrogen (BUN) and creatinine are not solely responsible for this symptom. Some proposed causes of uremic pruritus include the following:
-
Xerosis
-
Decreased transepidermal elimination of pruritogenic factors
-
Hyperparathyroidism
-
Hypercalcemia
-
Hyperphosphatemia
-
Elevated histamine levels
-
Increased dermal mast cell proliferation
-
Uremic sensory neuropathy
Therapeutic options, which are inconsistently helpful, include emollients to alleviate xerosis, augmentation of dialysis efficacy, normalization of serum calcium and phosphate levels, and parathyroidectomy. For some, sedating antihistamines and sauna therapy may provide temporary relief. Nonsedating antihistamines and topical steroids usually are not helpful.
Ultraviolet (UV) B phototherapy probably is the most effective therapeutic choice and may have prolonged benefit. Options such as cholestyramine and activated charcoal may decrease absorption of essential nutrients, perhaps making them more detrimental than beneficial. Although conflicting data exist, some clinical studies have suggested that opiate-receptor antagonists, such as naloxone and naltrexone, may ameliorate pruritus in some dialysis patients.
Pigmentary alteration
Pigmentary alteration occurs in 25-70% of the dialysis population and increases over time. A multitude of uremia-related changes are responsible for the pigmentary alterations. Before the widespread use of erythropoietin, pallor was common and was attributed to the significant anemia. A brown–to–slate-gray discoloration may occur as a result of hemosiderin deposition in association with iron overload from excessive transfusions. Over time, many patients develop a yellowish hue, which has been attributed to retained urochromes and carotene, which are subsequently deposited in the epidermis and subcutaneous tissues. A brownish hyperpigmentation is common, mostly in a sun-exposed distribution. This hyperpigmentation results from an increase in melanin production because of an increase in poorly dialyzable beta-melanocyte stimulating hormone.
Half-and-half nails
Bean first described half-and-half nails in 1963. [28] Although not pathognomonic for renal failure, these nails occur in as many as 40% of patients on dialysis and disappear several months after successful renal transplantation. Half-and-half nails are characterized by a dark distal band that occupies 20-60% of the nail bed and by a white proximal band. [29, 30, 31] The distal dark band may range in color from reddish to brown. Although the condition has been observed on toenails, it more commonly involves fingernails.
Alopecia
Alopecia probably is more common in end-stage renal disease (ESRD) than in the general population; however, alopecia has not been studied specifically in ESRD, and no reports exist regarding its prevalence or etiology. Likely causes of alopecia in ESRD include systemic lupus erythematosus (SLE) or chronic telogen effluvium. Chronic telogen effluvium may be related to the multitude and severity of illnesses encountered by patients, or it may be related to commonly used medications such as heparin, antihypertensives, or lipid-lowering agents.
Uremic frost
First described by Hirschsprung in 1865, uremic frost is rarely seen in the present day because of early dialytic intervention. When the blood urea nitrogen (BUN) level is adequately high (usually > 250-300 mg/dL), the concentration of urea in sweat is increased greatly. Evaporation results in the deposition of urea crystals on the skin. Uremic frost is commonly found in the beard or on other parts of the face, neck, and trunk as fine white-to-yellow crystals that dissolve readily when challenged by a drop of water.
Porphyria cutanea tarda
Plasma porphyrins are poorly dialyzed and are mildly to moderately elevated in most patients on dialysis. A bullous disease that is clinically similar to but metabolically distinct from porphyria cutanea tarda (PCT) has been described in the dialysis population (see Bullous Disease of Dialysis) (see the following image). [32]
 Hands of a transfusion-dependent patient on long-term hemodialysis. Several uremia-related cutaneous disorders are visible. The pigmentary alteration results from retained urochromes and hemosiderin deposition. The large bullae are consistent with either porphyria cutanea tarda or the bullous disease of dialysis. All nails show the distal brown-red and proximal white coloring of half-and-half nails.
Hands of a transfusion-dependent patient on long-term hemodialysis. Several uremia-related cutaneous disorders are visible. The pigmentary alteration results from retained urochromes and hemosiderin deposition. The large bullae are consistent with either porphyria cutanea tarda or the bullous disease of dialysis. All nails show the distal brown-red and proximal white coloring of half-and-half nails.
True PCT also has been reported to occur in patients on dialysis. Interestingly, because of other comorbid processes, an increased incidence of PCT should be expected to occur in end-stage renal disease (ESRD). Patients on dialysis are frequently recipients of blood transfusions because of uremia-related anemia. Excessive transfusions may result in considerable iron overload, which can contribute significantly to the development of PCT. Ferritin levels may reach the 1500-2000 ng/mL range in some patients.
Multiple transfusions also raise the rate of hepatitis C (HCV) infection. In the United States, the prevalence of HCV in the dialysis population is 8-36% compared with 0.8-1.2% in the general population. Worldwide prevalence of HCV in patients with PCT is 40-94%. [15] Although PCT is a cutaneous manifestation of HCV infection, exactly how HCV may trigger PCT remains undetermined.
Fortunately, the incidence of PCT in the dialysis population should decrease. Erythropoietin has decreased the number of transfusions patients require, and currently, blood products are screened routinely for HCV.
Arterial steal syndrome
The arterial steal syndrome is an uncommon but highly morbid complication of the vascular access necessary for hemodialysis. Production of an adequate vascular access requires the formation of an arteriovenous connection either by using native vessels (arteriovenous fistula) or by placing synthetic tubing (arteriovenous graft). Vascular access typically is placed in the nondominant upper extremity. See the image below.
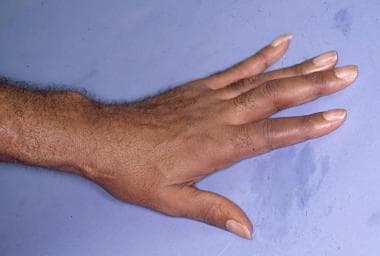 Arteriovenous graft visible on the patient's forearm. Cutaneous atrophy of the hand is the result of arterial steal syndrome resulting from this graft.
Arteriovenous graft visible on the patient's forearm. Cutaneous atrophy of the hand is the result of arterial steal syndrome resulting from this graft.
The arterial steal syndrome may develop if the inevitable proximal shunting of blood is significant enough to cause hand ischemia. [33] Proximal shunting is attributed to the reversal of blood flow through distal arteries, induced by the low-pressure system produced by the arteriovenous connection. Symptoms of arterial steal syndrome include pain and numbness. Prolonged ischemia may result in digital gangrene, peripheral neuropathy, or cutaneous atrophy. [33] Individuals at heightened risk for this complication include those with peripheral vascular disease, especially diabetes mellitus.
Dermatologic Disorders Associated With Renal Transplantation
Although many lives are saved and maintained by dialytic intervention, most individuals endure a great deal of morbidity as a result of the inadequacy of renal replacement therapy. The best therapeutic option for many patients with end-stage renal disease (ESRD) is renal allograft transplantation. Successful transplantation results in regression of many of the metabolic and cutaneous changes of uremia. The 2007 US Renal Data System (USRDS) report revealed that over 18,000 individuals were transplanted in 2006, raising the number of renal transplant recipients in the United States to over 140,000. Unfortunately, renal transplantation has its own set of complications, primarily resulting from the immunosuppressive medications that are essential for allograft survival.
Studies have shown that 50-100% of renal transplant recipients (RTRs) have a transplant-related cutaneous complaint. Dermatologic disorders associated with renal transplantation are a function of the immunosuppressive medications prescribed, as well as the immunosuppressed condition produced. Factors such as time after transplantation, geographic location, climate, and skin type greatly modify the clinical disorders associated with renal transplantation.
Medication-related dermatologic disorders
Medication-related disorders include the following:
-
Cushingoid changes
-
Gingival hyperplasia (see the following image)
-
Disorders of the pilosebaceous unit, including acne, folliculitis, hypertrichosis, keratosis pilaris, sebaceous gland hyperplasia, epidermal cysts (see the image below)
Many cutaneous changes seen in the renal transplant recipient (RTR) are related directly to medications used to suppress rejection of renal allograft. A full-blown Cushingoid appearance develops in 55-90% of patients and is associated with the high doses of corticosteroids used early after transplantation. Cutaneous findings include moon facies, development of a cervical fat pad (buffalo hump), striae distensae, cutaneous atrophy, and telangiectasias. Changes may resolve or improve when the corticosteroid dose is reduced, although these cutaneous changes may continue, as steroids are used long term. Gingival hyperplasia, which occurs in approximately one third of patients receiving cyclosporin A (CyA), tends to occur early and improve over time. See the image below.
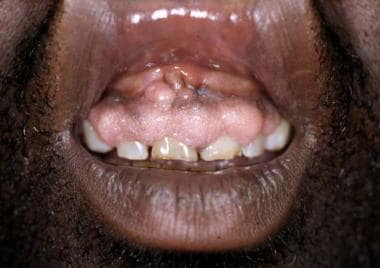 Cyclosporin may induce gingival hyperplasia in approximately one third of renal transplant recipients.
Cyclosporin may induce gingival hyperplasia in approximately one third of renal transplant recipients.
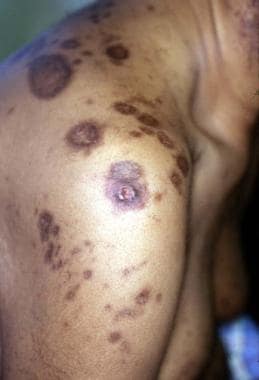
Other cutaneous changes involve poorly understood alterations in the pilosebaceous unit and may result from either CyA or corticosteroid use. Acne develops in 15% of patients and primarily affects the chest and back (see the image below). Most severe in the first year, acne later improves with reduction of the corticosteroid dose. Sebaceous gland hyperplasia and epidermal cysts are found with increased frequency and have been associated with the use of both corticosteroids and CyA. Hypertrichosis develops in 60% of patients and may be associated with the development of keratosis pilaris.
Immunosuppression-related disorders
Immunosuppression-related disorders include the following:
-
Viral infections, including herpes simplex virus (HSV), varicella-zoster virus (VZV), Epstein-Barr virus (EBV)
-
Bacterial infections, including Staphylococcus aureus, Bartonella henselae, mycobacteria, Mycobacterium tuberculosis, and atypical mycobacteria
-
Fungal infections, including superficial mycoses (eg, dermatophytes, Pityrosporum species, candidiasis) and deep fungal infections (eg, Aspergillus, Cryptococcus, Nocardia, Rhizopus species)
-
Parasitic infection, including scabies
-
Actinic keratoses
-
Malignancies, including squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, Kaposi sarcoma, melanoma
-
Miscellaneous malignancies, including lymphoma, Merkel cell carcinoma (MCC), and dermatofibrosarcoma protuberans
-
Miscellaneous disorders, including transfusion-associated graft-versus-host disease and porokeratosis
Infections
The iatrogenically induced decrease in cell-mediated immunity predisposes the renal transplant recipient (RTR) to infection by a variety of organisms. Timing and relative risk of the infections are determined by the degree of immunosuppression. Patients are at heightened risk for developing opportunistic infections during the first 6 months after transplantation because of the use of higher doses of immunosuppressive agents. Cutaneous examination is crucial in the surveillance for opportunistic infections, because cutaneous lesions frequently are the first sign of disseminated disease.
Mycobacterial infections
Later in the posttransplant period, patients may develop infections from a variety of acid-fast bacilli (AFB), specifically typical or atypical mycobacteria. [34, 35, 36] Although these infections are relatively unusual, they may cause significant morbidity. Multifocal disease is not uncommon. Organisms of the Mycobacterium fortuitum/chelonae complex are more common causes of AFB cutaneous infections, although others, such as M kansasii and M marinum, have also been reported. Histopathologic examination and tissue culture are necessary to make the correct diagnosis. Therapeutic options for these infections include antimicrobials, surgical debridement, and/or a reduction in immunosuppression.
Fungal infections
Fungal organisms are the most common cause of infection in the renal transplant recipient, occurring in 7-75%. [37] The wide variability in prevalence likely results from heterogeneity in diagnostic criteria, environmental exposures, geographic locations, and economic and hygienic factors.
Pityriasis versicolor has been shown to be the most common fungal infection and occurs in 18-48% of renal transplant recipients, which is a higher rate than found in the general population. Colonization of the upper back with Pityrosporum yeasts has been shown to occur 2-3 times more often in the renal transplant recipient relative to the general population. Pityrosporum organisms may predispose patients to increased incidence of folliculitis. Dermatophytosis, although common after renal transplantation, is no more common than in the general population.
Viral infections
Severe viral infections usually occur during the first year after transplantation and predominately result from herpes viruses. Cutaneous lesions resulting from infection with human papillomavirus (HPV) tend to develop later. Surveys suggest that the prevalence of HPV is 15-50% after the first year and increases to 77-95% by 5 years after transplantation.
Common and plane warts, which predominate, occur most frequently in sun-exposed areas and usually are multiple in number. HPV types 1, 2, 3, and 4 are found most commonly; however, many other HPV types have been reported in association with warty lesions in renal transplant recipients, including oncogenic types 16 and 18 and types 5 and 8, which usually are associated with epidermodysplasia verruciformis.
Eradication of these HPV infections is difficult, because the lesions respond poorly to therapy and recur frequently. Treating warts early and aggressively is best in the renal transplant recipient using routine modalities, such as cryotherapy, electrocoagulation, and carbon dioxide laser. Surgery and radiotherapy are not more effective and may result in scarring. Treatment with oral or topical retinoids may be an option for some patients.
Interferon alfa, which has been effective against warts in immunocompetent individuals, should not be used in the renal transplant recipient, because it may trigger allograft rejection. Imiquimod, an agent that can heighten the host's immune system by upregulating interferon, was initially thought to be similarly contraindicated in the transplant population. However, more recent studies have demonstrated it to be both effective and safe when used in a limited fashion.
Malignancy
Malignancies are more common after organ transplantation, and most are primary cutaneous malignancies. Incidence of nonmelanoma skin cancer is 20-40 times greater in renal transplant recipients (RTRs) than in the general population. This incidence increases as the time elapsed after transplantation increases because of the duration of immunosuppressive therapy. The cumulative risk of cutaneous malignancy is 10-30% at 5 years, 10-44% at 10 years, and 30-40% at 20 years. The prevalence of skin cancer varies according to geographic location, amount of ultraviolet (UV) light exposure, and predominant skin type. Most malignancies occur on sun-exposed areas and usually are found on the head, neck, and upper extremities. Multiple malignancies are a common occurrence.
Nonmelanoma skin cancer
Squamous cell carcinoma (SCC) is the most common cutaneous malignancy in renal transplant recipients and occurs 50-250 times more frequently in these individuals than in the general population. In contrast, basal cell carcinoma (BCC) occurs 6-10 times more frequently in the renal transplant recipient. As a result of the markedly increased incidence of SCC, an inversion is seen in the ratio of basal cell carcinoma to SCC, from 4:1 in the general population to 1:3-4 in the renal transplant population. The image below illustrates SCC.
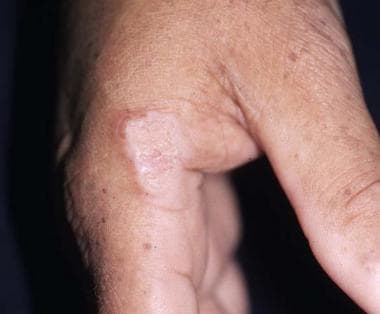 A hyperkeratotic plaque on this renal transplant recipient was proven to be squamous cell carcinoma. Similar lesions are frequently mistaken for warts.
A hyperkeratotic plaque on this renal transplant recipient was proven to be squamous cell carcinoma. Similar lesions are frequently mistaken for warts.
Nonmelanoma malignancies occur at a younger age in renal transplant recipients and are characterized by a more rapid and aggressive course, a higher recurrence rate, and a greater metastatic potential. Actinic keratoses also occur at a younger age and develop at a faster rate in renal transplant recipients. Actinic keratoses frequently have more severe cytologic atypia and may have more rapid progression to SCC. Clinically, it may be difficult to distinguish actinic keratoses, SCCs, and warts.
Factors that predispose individuals to development of nonmelanoma skin cancer include exposure to ultraviolet (UV) light, skin phototypes I and II, immunosuppression and, possibly, human papillomavirus (HPV) infection. Most cancers occur in sun-exposed areas in lightly pigmented individuals.
Immunosuppressive medications reduce cell-mediated immunity and, thereby, greatly augment the potential for malignant transformation. Whether cyclosporin A (CyA) is more or less oncogenic than azathioprine remains controversial, because available data are conflicting. Azathioprine and its metabolites are mutagenic and toxic to Langerhans cells, but CyA is more immunosuppressive.
Mounting evidence suggests that sirolimus-based immune suppression is associated with a much lower rate of skin cancer development in the solid organ transplant recipient. Many patients who are at high risk for skin cancer are being switched to this regimen; however. the risks and benefits of each agent must be considered and therapy customized to each patient. [38, 39, 40]
The role HPV may play remains obscure, because data are not conclusive; however, multiple HPV strains have been documented in many cutaneous malignancies, and malignant transformation of warty lesions has been observed.
Management of nonmelanoma skin cancers includes sun avoidance, use of broad-spectrum sunscreens, early detection of malignant and precancerous lesions, and aggressive therapy. Complete surgical excision with margin control is necessary. Adjuvant radiotherapy or chemotherapy may have a role in association with surgery in certain patients.
Some studies have shown imiquimod to be safe and effective for superficial BCCs and actinic keratoses when used on small surface areas according to directions [41, 42, 43, 44] ; however, the safety and efficacy of this agent in immunosuppressed patients have not been established. Some authors advocate switching from cyclosporin or tacrolimus to sirolimus in hope of minimizing cancer risk. Be aware that minimizing ultraviolet B (UVB) light exposure adds further risk for vitamin D deficiency in this population. [45, 46]
Chemoprevention using systemic retinoids should be reserved for those at highest risk for multiple malignancies. Partial and complete remissions have been reported with retinoid use, but long-term therapy is necessary, because the beneficial effect is lost 2-3 months after stopping treatment. [47, 48] Adverse effects may preclude retinoid use. Potential adverse effects include birth defects, hyperlipidemia, and osteoporosis. In addition, CS and CyA use frequently are associated with hyperlipidemia and osteoporosis; concern exists that these effects may be amplified by addition of a retinoid. Reduction or discontinuation of immunosuppressive therapy should be considered but may not be acceptable in some patients because it may result in allograft rejection and loss.
Kaposi sarcoma
The incidence of Kaposi sarcoma is 400-500 times higher in renal transplant recipients than in the healthy population. [17] Kaposi sarcoma accounts for approximately 3-5% of transplant-related malignancies in renal transplant recipients. The incidence varies according to geographic region and depends on ethnic composition of the population. Generally, Kaposi sarcoma incidence is higher in those with Jewish, Mediterranean, or Arabic ancestry and in blacks.
The highest incidence is in the first year after transplantation. Kaposi sarcoma usually appears 2-24 months after transplantation, in contrast to the late development of most other transplant-related malignancies. [49, 50, 51] As in other populations studied, the development of Kaposi sarcoma in renal transplant recipients is associated with human herpes virus 8 (HHV-8). Mucocutaneous lesions occur in 60% of renal transplant recipients with Kaposi sarcoma and may be isolated in these areas; however, visceral disease is not uncommon.
Therapeutic options for the renal transplant recipient with Kaposi sarcoma include cessation of immunosuppressive medications, radiotherapy, chemotherapy, excision, and cryotherapy. Although complete regression has been described after discontinuation of immunosuppressive therapy, adjuvant therapy often is needed. Occasionally, Kaposi sarcoma may be fatal as a result of visceral involvement and despite therapy.
Melanoma
Melanoma occurs 2-9 times more frequently in the transplant population than in the general population, according to population registry data and published studies. [52] Interestingly, the risk of melanoma in renal transplant recipient may include transmission by the donor. Studies are conflicting regarding the prognosis of melanoma in the transplant patient, including the risk of recurrence of melanoma following transplantation. [53, 54]
Other malignancies
Although Kaposi sarcoma is the most commonly reported sarcoma in renal transplant recipient, other cutaneous sarcomas have been reported. Malignant fibrous histiocytomas, atypical fibroxanthomas, and dermatofibrosarcoma protuberans have been reported in renal transplant recipients, although the incidence of these malignancies is unknown. Several cases of Merkel cell carcinoma (MCC) occurring in renal transplant recipients have been reported. [55] Most lesions develop on the head and neck or the extremities. This is an aggressive tumor with a high propensity to recur or metastasize. Overall mortality associated with Merkel cell carcinoma is 35%. Interestingly, reports exist of SCC and MCC occurring simultaneously.
Although lymphomas are the second most common malignancy in transplant recipients, cutaneous lymphomas are relatively rare. Whereas cutaneous T-cell lymphomas have been described, cutaneous B-cell lymphomas are more common in the transplant population. [56]
Miscellaneous disorders
Transfusion-associated graft-versus-host disease has been reported in several renal transplant patients who received nonradiated packed red blood cells. [57] Patients are at increased risk during times of marked immunosuppression, including therapy for acute organ rejection. Transfusion-associated graft-versus-host disease usually has a poor prognosis.
Porokeratosis, a disorder of epidermal keratinization, has been reported as an unusual manifestation of immunosuppression in solid organ recipients. [58, 59, 60] Several of the clinical variants have been reported, including disseminated superficial porokeratosis, single lesions of porokeratosis of Mibelli, and rare cases of disseminated porokeratosis of Mibelli. Malignant transformation of lesions of porokeratosis has been described but not in the transplant population.
Questions & Answers
Overview
What are the dermatologic manifestations of renal disease?
What is the role of dialysis in the dermatologic manifestations of end-stage renal disease (ESRD)?
How is diabetes mellitus associated with end-stage renal disease (ESRD)?
How is lupus erythematosus associated with end-stage renal disease (ESRD)?
How is granulomatosis with polyangiitis associated with end-stage renal disease (ESRD)?
How is polyarteritis nodosa associated with end-stage renal disease (ESRD)?
How are cholesterol emboli associated with end-stage renal disease (ESRD)?
How is hepatitis C virus (HCV) associated with end-stage renal disease (ESRD)?
How is HIV associated with end-stage renal disease (ESRD)?
How is systemic sclerosis associated with end-stage renal disease (ESRD)?
How is amyloidosis associated with end-stage renal disease (ESRD)?
What is the prevalence of xerosis in end-stage renal disease (ESRD)?
What causes xerosis in patients with end-stage renal disease (ESRD)?
What is the prevalence of pruritus in end-stage renal disease (ESRD)?
What causes pruritus in end-stage renal disease (ESRD)?
How is pruritus in end-stage renal disease (ESRD) treated?
What causes pigmentary alteration in end-stage renal disease (ESRD)?
What is the prevalence of half-and-half nails in end-stage renal disease (ESRD)?
What causes alopecia in end-stage renal disease (ESRD)?
What is uremic frost in end-stage renal disease (ESRD)?
What is the prevalence of porphyria cutanea tarda (PCT) in end-stage renal disease (ESRD)?
What causes arterial steal syndrome in end-stage renal disease (ESRD)?
Which dermatologic disorders are associated with renal transplantation?
What are the medication-related dermatologic disorders associated with renal transplantation?
What are the immunosuppression-related dermatologic disorders associated with renal transplantation?
What are the infection-related dermatologic disorders associated with renal transplantation?
What is the prevalence of cutaneous malignancies following renal transplantation?
What is the prevalence of nonmelanoma malignancies following renal transplantation?
What causes nonmelanoma malignancies following renal transplantation?
How are nonmelanoma malignancies treated in patients with renal transplantation?
What is the prevalence of Kaposi sarcoma (KS) following renal transplantation?
How is Kaposi sarcoma (KS) treated in patients with renal transplantation?
What is the prevalence of melanoma following renal transplantation?
Which cutaneous malignancies have been reported following renal transplantation?
What causes transfusion-associated graft-versus-host disease following renal transplantation?
What causes porokeratosis following renal transplantation?
-
Hands of a transfusion-dependent patient on long-term hemodialysis. Several uremia-related cutaneous disorders are visible. The pigmentary alteration results from retained urochromes and hemosiderin deposition. The large bullae are consistent with either porphyria cutanea tarda or the bullous disease of dialysis. All nails show the distal brown-red and proximal white coloring of half-and-half nails.
-
Chronic scratching resulting from uremic pruritus may result in numerous cutaneous lesions. This patient has many atrophic hyperpigmented scars and an excoriated prurigo nodule on the shoulder.
-
Patients with uremia may develop an acquired perforating disorder. These highly pruritic, hyperkeratotic papules of Kyrle disease are present on the lower extremity of a patient with diabetes and end-stage renal disease.
-
Arteriovenous graft visible on the patient's forearm. Cutaneous atrophy of the hand is the result of arterial steal syndrome resulting from this graft.
-
Lesions of steroid-induced acne (evident on the back of a renal transplant patient) may be severe.
-
Cyclosporin may induce gingival hyperplasia in approximately one third of renal transplant recipients.
-
Follicular prominence and keratosis follicularis may be seen in renal transplant recipients because of use of immunosuppressive agents.
-
A hyperkeratotic plaque on this renal transplant recipient was proven to be squamous cell carcinoma. Similar lesions are frequently mistaken for warts.