Background
Anhalt et al [1] first described paraneoplastic pemphigus in 1990. The authors reported five patients with underlying neoplasms who developed oral erosions and bullous skin eruptions. Skin biopsy samples showed both suprabasal acantholysis and interface dermatitis. Direct immunofluorescence (DIF) testing and indirect immunofluorescence (IDIF) testing revealed intraepidermal intercellular staining with immunoglobulin G (IgG); DIF testing also revealed deposition of complement at the dermoepidermal junction (see the image below). By immunoprecipitation, target antigens were identified from skin extracts with molecular weights of 250, 230, 210, and 190 kd. Since then, many patients with paraneoplastic pemphigus have been reported, and patients previously believed to have other diseases have been retrospectively diagnosed.
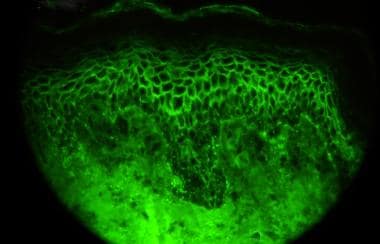 Direct immunofluorescence microscopy performed on epithelial biopsy specimen obtained from a patient with pemphigus vulgaris detects immunoglobulin G deposits at the epithelial cell surfaces.
Direct immunofluorescence microscopy performed on epithelial biopsy specimen obtained from a patient with pemphigus vulgaris detects immunoglobulin G deposits at the epithelial cell surfaces.
A summary of the original criteria for the diagnosis of paraneoplastic pemphigus includes the following:
-
Painful mucosal erosions, sometimes with a skin eruption that eventually results in blisters and erosions, in the setting of confirmed or occult malignancy
-
Histopathologic changes of acantholysis, keratinocyte necrosis, and interface dermatitis
-
DIF observation of immunoreactants, typically IgG and complement (C3) within the epidermal intercellular spaces as well as at the epidermal basement membrane
-
IDIF observation of circulating antibodies specific for stratified squamous or transitional epithelia (transitional epithelium)
-
Immunoprecipitation of a complex of proteins with typical molecular weights, as described in Other Tests
Because not all patients demonstrate these original criteria, Anhalt [2] has proposed the following new, minimal criteria for the diagnosis of paraneoplastic pemphigus:
-
Painful, progressive stomatitis
-
Histopathologic changes of acantholysis or lichenoid/interface dermatitis
-
Demonstration of antiplakin antibodies
-
Demonstration of an underlying lymphoproliferative neoplasm
Paraneoplastic pemphigus is rare, and, while the exact incidence is unknown, it is likely underreported. In 2010, 450 cases were reported in the literature. [3] There is an association between paraneoplastic pemphigus and HLA class II DRB I*03 and HLA-Cw*14 (in Chinese patients). [4, 5]
Hematologic malignancies are most commonly associated with paraneoplastic pemphigus, although it can also be associated with carcinomas, sarcomas, and benign neoplasms.
Unlike other forms of pemphigus, paraneoplastic pemphigus affects organ systems other than the integument. Thus, the term "paraneoplastic autoimmune multiorgan syndrome", or "PAMS", has been suggested as a more appropriate name for the syndrome. [6]
Pathophysiology
Paraneoplastic pemphigus is an autoimmune disorder initiated by an underlying neoplasm. Tumor antigens are hypothesized to evoke both a humoral and a cellular immune response that leads to blistering in mucosa and other epithelia. Affected organ systems include the integument, respiratory tract, and gastrointestinal tract. A patient with renal and neurologic involvement has been reported. [7]
Passive transfer of paraneoplastic pemphigus sera causes blistering in neonatal mice, proving that the antibodies present are pathogenic. [1] Paraneoplastic pemphigus patients exhibit many different autoantibodies to proteins of the plakin family, an intracellular component of desmosomes and hemidesmosomes, including envoplakin (210-kd), periplakin (190-kd), bullous pemphigoid antigen I (230-kd), desmoplakin I (250-kd), desmoplakin II (210-kd), plectin (500-kd), and alpha2-macroglobulin-like–1 (170-kd). They can also exhibit antibodies to antigens associated with pemphigus vulgaris (desmoglein 3, 130-kd) and pemphigus foliaceus (desmoglein 1, 160-kd), as well as several others, including epiplakin. [8] Antibodies are typically IgG, although IgA has been reported. [9]
In a 2011 review, Czernik et al. summarize the increasing role of cellular immunity in paraneoplastic pemphigus, evidenced by lesional mononuclear cells and elevated IL-6 levels in sera from paraneoplastic pemphigus patients. [6] Patients with paraneoplastic pemphigus without identifiable autoantibodies have been reported, [10, 11, 12] as has a patient with lichenoid paraneoplastic pemphigus who lacked autoantibodies initially but developed them later in their course. [13] It has been postulated that the diagnosis of paraneoplastic pemphigus is declining, owing to treatment of the associated malignancies with anti-CD20 antibodies, which reduce the humoral response and lead to unrecognized T-cell–mediated lichenoid eruptions. [14]
Etiology
The association of paraneoplastic pemphigus with malignancy is strong. Patients have had benign neoplasms, including thymoma and Castleman disease. Castleman disease is a common association in children. Only a single patient without a tumor has met the diagnostic criteria, yet this patient had a rapid demise and may have died with an undiagnosed malignancy. Patients have developed paraneoplastic pemphigus while in remission of their malignancy, leading some authors to prefer the term neoplasia-induced pemphigus. [15]
Treatment of the underlying malignancy does not halt progression of the paraneoplastic pemphigus, especially if the surgery is not curative or if pulmonary manifestations (end-organ disease) are present. Cases associated with benign neoplasms can improve dramatically when the tumor is resected, owing to the decreased production of autoantibodies. [16]
Circulating and tissue-bound antibodies in patients with paraneoplastic pemphigus are directed against cadherins and a group of molecules with sequence homology and belonging to the plakin family. Plakins are found in the intracellular attachment plaques of desmosomes and hemidesmosomes, and they play a key role in intermediate filament attachment. However, the number of reported target antigens has increased over time and varies between patients. This variability likely accounts for the clinical heterogeneity of this disease. By immunoprecipitation, target antigens (in decreasing order of incidence) include desmoglein 3, desmoglein 1, envoplakin, periplakin, desmoplakin I, desmoplakin II, and bullous pemphigoid antigen I. Plectin, a 170-kd protein called alpha2-macroglobulin-like–1, and epiplakin have also been found. [17, 18, 8]
How tumors induce autoantibodies to plakin proteins is not known. Tumor cells have been demonstrated to produce autoantibodies that react to epidermal proteins. Other postulates include (1) cross-reactivity of tumor antigens and epidermal antigens and (2) tumor production of plakin proteins that initiate an autoimmune response. Dysregulated cytokine production by tumor cells, specifically interleukin 6, contributing to the autoimmune process is another hypothesis. The concept of epitope spreading, with which patients develop antibodies to multiple structurally related and unrelated proteins, may explain the multitude of antibodies produced in association with this disease.
Epidemiology
Race
No racial predilection is apparent for paraneoplastic pemphigus.
Sex
Males are affected more than females. [6]
Age
Paraneoplastic pemphigus has been reported in patients aged 7-83 years. It typically affects patients aged 45-70 years, but it can also be seen in children and adolescents. [16]
Prognosis
The prognosis of paraneoplastic pemphigus is poor. High mortality rates are due to systemic complications, and treatment of the underlying tumor does not necessarily improve prognosis.
The mortality rate when associated with malignancy is estimated at 90%. Nearly all patients with the 2 most common associated tumors, non-Hodgkin lymphoma and chronic lymphocytic lymphoma, are dead of disease within 2 years of diagnosis. Note, however, that outcome does not parallel the course of the underlying malignancy. Both the presence of an underlying neoplasm and the adverse effects of the potent medications required to treat the disease add to both the morbidity and the mortality.
Paraneoplastic pemphigus is the only form of pemphigus that affects epithelia other than squamous. Involvement of respiratory mucosa, which manifests clinically as dyspnea with normal chest radiograph findings, is an ominous finding that progresses via an unknown mechanism to bronchiolitis obliterans. The most recent estimates are that approximately one third of the deaths from paraneoplastic pemphigus are due to pulmonary insufficiency.
Mortality/morbidity
Paraneoplastic pemphigus is often fatal, especially when associated with malignancy. Mortality rates approach 90%. Causes of death include the evolution of the underlying neoplasm, sepsis with resultant multiorgan failure, and respiratory failure due to the direct effects of the disease on the respiratory tract epithelium. The latter is being increasingly recognized, and pulmonary involvement has been found to occur in approximately 30-40% of patients. Bronchiolitis obliterans is characteristic. The susceptibility to infection caused by the loss of skin integrity is exacerbated by the potent immunosuppressive medications used to treat the condition.
Patients with paraneoplastic pemphigus and long-term survival have been described. Those whose underlying neoplasm is benign, such as in Castleman disease and thymoma, tend to have a better prognosis.
A 2012 study on 53 patients with paraneoplastic pemphigus found a mortality rate of 68%. [19] The authors surmise that this was lower than other reported rates because of the inclusion of patients with only moderate, as opposed to severe, disease. Patients with erythema multiforme–like lesions and keratinocyte necrosis on biopsy, especially when associated with severe skin or mucosal involvement, tended to have a worse prognosis.
Patient Education
As with any serious illness, patients should be made aware of the poor prognosis. For patient education resources, see the Procedures center, as well as Skin Biopsy.
-
Direct immunofluorescence microscopy performed on epithelial biopsy specimen obtained from a patient with pemphigus vulgaris detects immunoglobulin G deposits at the epithelial cell surfaces.




