Practice Essentials
Perhaps nothing is more gratifying for cosmetic patients than having an immediate correction of rhytides or scars as a result of the injection of a dermal filler. Before and after photos of patients who have had injection of dermal fillers are shown below.
Since its approval in 1981, bovine collagen had been the only US Food and Drug Administration (FDA)–approved dermal filler more than a decade. Quickly, this dermal filler gained popularity and was marketed under the name Zyderm I. [1] This dermal filler was found to be extremely effective for the correction of fine lines and shallow scars, with results often lasting 3 months.
Moderate rhytides, deeper nasolabial folds, and marionette lines could be treated using Zyderm I with fair results, but results often did not last more than 2 months. What became clear is that Zyderm I had 3 major shortcomings: (1) the potential for allergy to the bovine collagen, which required skin testing prior to the first treatment; (2) results generally lasted 3 months or less; and (3) disappointing results if used to fill moderate rhytides, skin folds, or scars.
Two years later, Zyderm II and Zyplast were FDA approved and introduced into the market to address the latter 2 shortcomings of Zyderm I. These newer dermal fillers, Zyplast in particular, significantly improved overall results for deeper rhytides and folds, and these dermal fillers, for more than a decade, remained the only dermal fillers that were FDA approved for use in the United States. Despite this, the need for most patients to have frequent treatments, the need for new patients to undergo skin allergy testing prior to treatment, and the limitations of the treatment of deeper rhytides and folds, meant the full potential for dermal fillers was not seen until the recent addition of newer dermal fillers to the armamentarium.
The perfect dermal filler would be inexpensive, safe, painless to inject, hypoallergenic, and long lasting. In addition, it should have consistent and predictable results, feel natural under the skin, take little time to inject, be ready-to-use, cause no downtime for the patient, and have a low risk of complications. With the increasing desire for people to achieve a more youthful appearance, the aging baby boomer population, and the increased demand for "lunch-time procedures," the pharmaceutical market has responded by providing the cosmetic surgeon with an increasing number of options to meet the demands of the cosmetic patient. Thus, this segment of cosmetic surgery has been the fastest growing for the past decade.
By definition, a dermal filler is a product that is injected or placed into the dermis. Patients are instructed to not manipulate the treated areas, because the product may shift. The best way to reduce inflammation is to immediately apply a cool pack to the areas that were treated. In current practice, several dermal fillers are available for use in the United States, in addition to subdermal fillers, or those that are placed underneath the dermis in the subcutis.
This article primarily addresses the dermal fillers that are FDA approved for use in the United States. Nonanimal, animal, and synthetic dermal fillers are mentioned in the article. Cadaveric-derived dermal fillers and implants are not mentioned because they are costly, not all of them are FDA approved, and they are used more frequently for burn victims.
Caution
In May 2015, the FDA issued a warning to healthcare providers and the public about serious complications that can occur if dermal fillers are inadvertently injected into blood vessels in the face. [2] The complications could possibly include vision impairment, blindness, stroke, and damage and/or necrosis of the skin and underlying facial structures. Caution should be used to ensure proper placement of the filler material, and patients should be informed about the potential adverse effects and how to recognize symptoms of impending serious complications.
Additionally, there has been at least 1 reported case of herpes zoster reactivation after hyaluronic acid filler injection to the nose. [3]
Filler migration may occur with dermal filler treatment. Migration may not manifest until many months after treatment. Healthcare providers should be aware of the possibility of filler migration, especially when evaluating forehead or ocular orbit masses. [4] There is at least 1 report of filler migration mimicking a parotid mass. [5]
COVID-19 and dermal fillers
There have been reported cases of delayed local reactions to hyaluronic acid dermal filler injections in patients with previous COVID-19 infections. In 1 case, the localized reaction did not manifest until 10 months after hyaluronic acid dermal filler treatment. Additionally, hypersensitivity reactions have been reported in patients who received COVID-19 vaccination and subsequent hyaluronic acid dermal filler treatment. Such reactions have occurred after vaccination with the Pfizer‐BioNTech COVID‐19 vaccine as well as the Moderna COVID-19 vaccine. [6, 7, 8, 9]
Hyaluronic Acid
Hyaluronic acid is the most prominent glycosaminoglycan in the skin. Hyaluronic acid potently binds to water and, when injected into the skin, volumizes, softens, and hydrates the skin. In addition to these benefits, it plays a role in cell growth, membrane receptor function, and adhesion.
Hyaluronic acid stabilizes intercellular structures and produces the viscoelastic network for collagen and elastin fibers to bind together. As seen with photoaging, these connections fail, thus resulting in disorganized clumps of collagen and elastin. These benefits make hyaluronic acid an excellent dermal filling agent. [10, 11] In February 2003, the FDA approved Restylane, a cross-linked, nonanimal source hyaluronic acid. This dermal filler was quickly found to be relatively long lasting, have minimal adverse effects, was easy to use, was ready to use out of the box, did not require refrigeration, was cost effective, and did not require skin testing prior to treatment.
Because hyaluronic acid is identical in all species, the risk of allergy is remote. Hyaluronic acid has a heparinlike effect, thus resulting in a greater incidence of bruising than is seen with collagen fillers. [12] Until 2010, nearly every FDA-approved hyaluronic acid product in the United States did not contain lidocaine, thus significantly increasing the discomfort experienced with injection of these dermal fillers compared with the bovine- and human-derived collagen fillers. Despite these shortcomings, hyaluronic acid fillers still emerged as the leader of dermal filling agents for soft tissue augmentation, owing to their superior cosmetic results.
In the uncommon circumstance when an undesirable outcome occurs with hyaluronic acid, correction is possible with the injection of commercially available hyaluronidase, which breaks down the unwanted hyaluronic acid dermal filler. The use of hyaluronidase for this purpose is not FDA approved and is considered an off-label use. In many cases, 10-30 units of unpreserved hyaluronidase is sufficient to achieve the desired correction. Local site reactions may occur in up to 25% of persons, although they are typically transient and mild. Initial treatment with as little as 5-10 units is commonly recommended and is often effective, although some treat with as much as 75 units with few adverse effects. Additional corrections can be performed, although full correction may take up to 4 weeks to fully appreciate. Some preparations are bovine derived, and skin testing should be considered prior to treatment with these dermal fillers. [13, 14]
Hyaluronidase preparations are clear, concentrated liquids that are stored in a refrigerated vial. To reconstitute these products, physicians typically add normal saline or lidocaine (with or without epinephrine). When using Amphadase, reconstitution using 3 mL of 1% lidocaine with 1:100,000 epinephrine has been commonly used with great success. After mixing, the vial is gently swirled. Prior to treatment, a skin test can be performed by injecting 3-5 units (0.06-0.1 mL) of the reconstituted solution into the superficial dermis at the antecubital fossa. A positive hypersensitivity reaction consists of a wheal appearing within 5 minutes and lasting 20-30 minutes, accompanied by local itching. [13]
Restylane
Restylane and Restylane-L
Restylane was approved by the FDA in 2003 for the treatment of nasolabial folds. This dermal filler is produced by fermentation in bacterial cultures of equine streptococci and contains approximately 100,000 particles per mL (20 mg/mL). These particles are approximately 300 microns and are highly cross-linked (single cross-linked) using an ether bond, making these dermal fillers one of the stiffest hyaluronic acid fillers. This dermal filler has been used for correction of the nasolabial folds, marionette lines, tear troughs, and glabellar frown lines, in addition to lip enhancement and cheek augmentation. Other clinical uses include correction of the jowls and nasal deformities. In general, most patients can expect 6 months of correction, if not longer. [15] In 2010, Restylane-L became available, which contains lidocaine to reduce pain upon injection. Before and after photos of Restylane injection are shown below.
Restylane Lyft with lidocaine
This dermal filler was originally marketed as Perlane-L, but had a name change in 2015. Restylane Lyft is identical to Restylane except that it consists of larger gel particles that are approximately 1000 microns. There are approximately 20,000 particles per mL. This dermal filler is suitable for the correction of deeper folds, such as the nasolabial folds, and works well for cheek enhancement and temporal recession. Additionally, some experienced physicians prefer this dermal filler over Restylane for lip augmentation, although due to the stiffness of the product this is not common. Most patients can expect 6-12 months of correction with Perlane. In 2010, Perlane-L became available, which contains lidocaine to reduce pain upon injection. Before and after photos of Perlane injection are shown below.
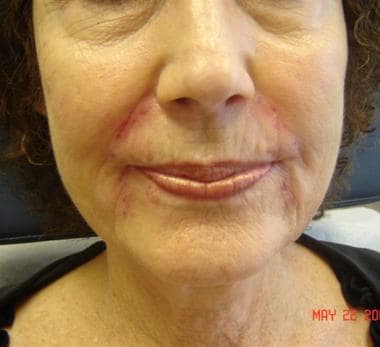 Patient 7. After photo. Dr. Bader injected Perlane (1 mL) into nasolabial folds, upper and lower lips, and marionette lines.
Patient 7. After photo. Dr. Bader injected Perlane (1 mL) into nasolabial folds, upper and lower lips, and marionette lines.
Restylane Silk
This dermal filler was FDA approved in 2014 for submucosal implantation for lip augmentation and dermal implantation for correction of perioral rhytids in patients older than 21 years. It has the same concentration of hyaluronic acid and a pH of 7.0 as the other Restylane products, but the molecules are smaller. There are 500,000 particles per mL. This product is intended to be used for fine perioral lines, although other areas on the face can be treated as well.
Restylane Refyne
This dermal filler was FDA approved in 2016 for the treatment of moderate to severe facial wrinkles and folds in patients older than 21 years. It has the same concentration of hyaluronic acid and a pH of 7.0 as the other Restylane products, but is more pliable than Restylane or Restylane Lyft.
Restylane Defyne
This dermal filler was FDA approved in 2016 for the treatment of moderate to severe, deep facial wrinkles and folds in patients older than 21 years. It has the same concentration of hyaluronic acid and a pH of 7.0 as the other Restylane products, but is more pliable than Restylane or Restylane Lyft.
Restylane Kysse
This dermal filler was FDA approved in 2020 for submucosal implantation for lip augmentation and dermal implantation for correction of perioral rhytids in patients older than 21 years. It has the same concentration of hyaluronic acid and pH of 7.0 as the other Restylane products.
Restylane Contour
This dermal filler was FDA approved in 2021 for cheek augmentation and correction of midface contour deficiencies in patients over the age of 21. dermal implantation for correction of perioral rhytids in patients older than 21 years. It has the same concentration of hyaluronic acid and a pH of 7.0 as the other Restylane products.
Precautions
Use of products that contain lidocaine reduces pain upon injection and is recommended, unless contraindications to the use of lidocaine are present. Erythema and edema are common after treatment and typically last a few days. When injected too superficially, a bluish Tyndall effect can be seen, which represents visible hyaluronic acid seen through the translucent epidermis. Fortunately, these bluish cysts are easily corrected by nicking the skin with a small-gauge needle (30 gauge) or No. 11 blade and expressing the superficial, unwanted dermal filler. [16]
Occasionally, palpable nodules can be felt under the skin, which often occurs when the multiple-puncture technique is used or depot injections are performed to place the dermal filler. For this reason, linear threading is now commonly used, which minimizes the risk of nodules. Some prefer using a small (30-gauge) blunt-tipped cannula to inject instead of the 30-gauge needle that comes with the product. As with other fillers, patients should avoid all blood thinners for 10 days prior to treatment. [17] Implantation into a blood vessel may result in vascular occlusion, infarction, or embolic phenomena.
Table 1. Nonanimal Stabilized Hyaluronic Acid (Open Table in a new window)
Agent |
Contents |
Method of Action |
Indications |
Injection |
Duration |
Limits |
Restylane and Restylane-L |
Medium-sized particles of stabilized hyaluronic acid generated by streptococcal bacteria and formulated to a concentration of 20 mg/mL and suspended in a physiological buffer at a pH of 7.0. |
Adds natural volume as it integrates into the dermal tissue; then, attracts and binds water molecules to help maintain volume. |
Mid-to-deep dermal implantation for correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds; submucosal implantation for lip augmentation in patients older than 21 years. |
Supplied in a disposable glass syringe; each syringe contains 0.4 mL, 1 mL, or 2 mL gel for injection into mid dermis. |
Approximately 6 months. |
20 mL/60 kg (130 lb) body mass per year. |
Restylane Lyft with lidocaine |
Hyaluronic acid chemically cross-linked with BDDE, pH = 7.0 and concentration of 20 mg/mL; largest fraction of gel particles size is 940-1090 µm. |
Adds natural volume as it integrates into the deep dermal tissue or subcutis; then, attracts and binds water molecules to help maintain volume. |
Implantation into deep dermis to superficial subcutis for correction of moderate-to-severe facial folds and wrinkles, such as nasolabial folds, or in patients older than 21 years who have age-related volume loss. |
Supplied in 1-mL glass syringes for injection; injected into mid-to-deep dermis or supraperiosteal. |
Approximately 6-12 months. |
20 mL/60 kg (130 lb) body mass per year. |
| Restylane Silk | Same hyaluronic acid concentration as Restylane, but composed of smaller gel particles and contains 0.3% lidocaine. | Adds natural volume as it integrates into the dermal tissue; then, attracts and binds water molecules to help maintain volume. | Submucosal implantation for lip augmentation and dermal implantation for correction of perioral rhytids in patients older than 21 years. | Supplied in a disposable glass syringe; each syringe contains 1 mL for injection into mid dermis. | Approximately 6 months. | The recommended maximum injected volume per treatment session is 3mL as greater amounts increases the risk of treatment site reactions. |
| Restylane Refyne | The product has a sodium hyaluronate concentration of 20 mg/mL in phosphate buffered saline at pH 7 and contains 3 mg/mL lidocaine hydrochloride. | Adds natural volume as it integrates into the dermal tissue; then, attracts and binds water molecules to help maintain volume. | Restylane Refyne is indicated for injection into the mid-to-deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds) in patients over the age of 21. | Supplied in 1-mL glass syringes for injection; injected into mid-to-deep dermis | Up to 1 year. | |
| Restylane Defyne | Adds natural volume as it integrates into the deep dermal tissue or subcutis; then, attracts and binds water molecules to help maintain volume. | Mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds. Restylane® Defyne is also indicated for injection into the mid-to-deep dermis (subcutaneous and/or supraperiosteal) for augmentation of the chin region to improve the chin profile in patients with mild to moderate chin retrusion. | Supplied in 1-mL glass syringes for injection; injected into mid-to-deep dermis or supraperiosteal. | Up to one year. | ||
| Restylane Contour | Cheek augmentation and for the correction of midface contour deficiencies. | The recommended maximum injected volume per treatment session is 6mL | ||||
| Restylane Kysse | Sodium hyaluronate with a concentration of 20 mg/mL that is crosslinked with BDDE (1,4-butanediol diglycidylether) in phosphate buffered saline at pH 7 and contains 3 mg/mL lidocaine hydrochloride. | Adds natural volume as it integrates into the deep dermal tissue or subcutis; then, attracts and binds water molecules to help maintain volume. | lip augmentation and for correction of upper perioral wrinkles in patients over the age of 21. | Supplied in 1-mL glass syringes for injection; injected into the submucosa or the mid-to-deep dermis. | Up to one year, although some have longer results. | Recommended maximum injected volume per treatment session is 3mL for the lips and 3mL for the perioral area. |
Juvederm (Juvederm Voluma XC, Juvederm Vollure XC, Juvederm Ultra Plus XC, Juvederm Ultra XC, Juvederm Volbella XC)
In 2006, the FDA approved Juvederm, which is also a nonanimal stabilized hyaluronic dermal filler. In the United States, five types of Juvederm dermal fillers are FDA approved. Juvederm Ultra Plus contain 24 mg/mL of hyaluronic acid, but Juvederm Ultra Plus XC has a higher proportion of cross-linking than Juvederm Ultra XC. Juvederm is a homologous gel with the highest degree of cross-linking of any of the hyaluronic acid fillers and thus has a smooth consistency. [18] Juvederm Ultra XC and Juvederm Ultra Plus XC have indications similar to those of Restylane and Restylane Lyft, respectively, and also do not require refrigeration or skin tests prior to use. In 2010, both Juvederm Ultra and Juvederm Ultra Plus became available with lidocaine, to reduce pain with injection, both labeled as Juvederm (R), and now carfy the XC designation.
Precautions
The adverse effects of Juvederm are similar to those seen with Restylane and Perlane. [19] Like all of the hyaluronic acid filling agents that are FDA approved, Juvederm does not contain lidocaine. Therefore, in addition to discomfort with injection, one may see erythema, swelling, and bruising. If injected too superficially, a bluish Tyndall effect and nodules may appear. [20]
Table 2. Juvederm Dermal Fillers (Open Table in a new window)
Agent |
Contents |
Method of Action |
Indications |
Injection |
Duration |
Limits |
Juvederm Ultra XC |
Sterile, biodegradable, nonpyrogenic, viscoelastic, clear, colorless homogenized gel implant; cross-linked hyaluronic acid formulated to a concentration of 24 mg/mL suspended in a physiological buffer; hyaluronic acid is produced by Streptococcus equi bacteria. Juvederm Ultra XC contains 0.3% lidocaine. |
Adds natural volume as it integrates into dermal tissue; then, attracts and binds water molecules to help maintain volume. |
Mid-to-deep dermis for correction of moderate-to-severe facial wrinkles and folds (eg, nasolabial folds); Juvederm Ultra XC is indicated for injection into the lips and perioral area for lip augmentation in adults older than 21 years. |
One box contains 2 prefilled syringes, each containing 0.8 mL of hyaluronic acid; inject into mid dermis. |
Approximately 6-12 months. |
20 mL/60 kg (130 lb) body mass per year. |
Juvederm Ultra Plus XC |
24 mg/mL of hyaluronic acid (same as above) but with a higher proportion (11%) of cross-linked hyaluronic acid. Juvederm Ultra Plus XC contains 0.3% lidocaine. |
Same as above. |
Same as above. |
Same as above. Injected into mid-to-deep dermis. |
Approximately 6-12 months. |
20 mL/60 kg (130 lb) body mass per year. |
| Juvederm Voluma XC | It is a sterile, biodegradable, non-pyrogenic, viscoelastic, clear, colorless, homogenized gel implant. It consists of crosslinked hyaluronic acid (HA) produced by Streptococcus equi bacteria, formulated to a concentration of 20 mg/mL and 0.3% w/w lidocaine in a physiologic buffer. | Adds natural volume as it integrates into the subcutis; then, attracts and binds water molecules to help maintain volume. | Deep (subcutaneous and/or supraperiosteal) injection for cheek augmentation to correct age-related volume deficit in the mid face and for chin region to improve the chin profile in adults older than 21 years. | Injected subdermally or above the periosteum. | Approximately 9-12 months. | 20 mL/60 kg (130 lb) body mass per year. |
| Juvederm Vollure XC | An injectable gel is manufactured using VYCROSS® technology formulated to a concentration of 17.5 mg/mL hylauronic acid and 0.3% w/w lidocaine in a physiologic buffer. | Adds natural volume as it integrates into dermal tissue; then, attracts and binds water molecules to help maintain volume. | correction of moderate to severe facial wrinkles and folds, such as nasolabial folds in patients over 21 years.. | Injected into mid-to-deep dermis | Approximately 6 - 12 months, but can last 18 months. | 20mL/60kg per year. |
| Juvederm Volbella XC | Hyaluronic acid 15mg/mL, 0.3% w/w lidocaine; injectable gel implant. | Adds natural volume as it integrates into dermal tissue; then, attracts and binds water molecules to help maintain volume. | lip augmentation and correction of perioral lines, and for injection into the undereye hollows to improve the appearance of undereye hollows in adults over the age of 21. | Injected into the superficial to mid dermis for the correction of perioral lines or above the periosteum for hollows under the eyes. | Approximately 6-12 months. | 20mL/60kg per year. |
Belotero Balance
This product was first approved for use in the United States in 2011. It is a hyaluronic acid-based cohesive gel dermal filler approved to temporarily smooth out and fill in moderate to- severe nasolabial folds (the folds or wrinkles that go from the side of the nose to the corner of the mouth).
Precautions
The adverse effects are similar to the other hyaluronic fillers.
Table. (Open Table in a new window)
| Belotero Balance |
Table. (Open Table in a new window)
Agent |
Contents |
Method of Action |
Indications |
Injection |
Duration |
Limits |
Table. (Open Table in a new window)
| XC | A bacterially fermented, injectable, hyaluronic-acid-based dermal filler. After extraction and purification, hyaluronic acid manufactured from streptococcal cultures is cross-linked with a binding agent 1,4-butanediol diglycidyl ether (BDDE) in two consecutively executed reactions and reconstituted in a physiologic buffer at pH 7 and concentration of 22.5 mg/mL. | Adds natural volume as it integrates into dermal tissue; then, attracts and binds water molecules to help maintain volume. | Approved to temporarily smooth out and fill in moderate to- severe nasolabial folds, but has been used off label to treat perioral rhytids, hollows under the eyes, and lip augmentation | Injected into the mid dermis. | Approximately 6 months or more. | 6ml/year |
Polymethylmethacrylate With Bovine Collagen
Bellafill (previoulsy called Artefill) was FDA approved for use in the United States in 2007. This dermal filler is composed of nonresorbable polymethylmethacrylate (PMMA) microspheres, which are 30-50 μ m in diameter, suspended in a water-based carrier gel composed of 3.5% bovine collagen, 92.6% buffered isotonic water, 0.3% lidocaine, 2.7% phosphate buffer, and 0.9% sodium chloride. [21] Because this dermal filler contains lidocaine, the injection is less painful compared with other dermal fillers that do not contain lidocaine. This dermal filler is indicated for the correction of the nasolabial folds, although it has been used for acne scars and forehead furrows. Using a 26-gauge needle, Bellafill should be injected directly beneath the skin fold into the deep dermis and not into the subcutis. Most practitioners prefer a threading injection technique. Unlike the other dermal fillers, this dermal filler should be considered a permanentdermal filler. [22]
Precautions
Because this product contains bovine collagen, skin testing must be performed prior to treatment, as would be indicated with Zyderm or Zyplast (see Collagen). Because results are permanent, it is often best not to try to achieve full correction in one session, but to accomplish the desired result over several treatment sessions. In addition to allergy, other adverse effects may include lumpiness, persistent swelling or redness, and increased sensitivity at the injection site. [23] As the results are permanent, any imperfections seen after treatment will remain. The product must be refrigerated.
Table 7. Bovine-Derived Nonresorbable Implant (Open Table in a new window)
Agent |
Contents |
Method of Action |
Indications |
Injection |
Duration |
Limits |
Bellafill (previously Artefill) |
Composed of PMMA microspheres (diameter 30-50 μ m) suspended in a water-based carrier gel containing 3.5% purified bovine collagen, 92.6% buffered isotonic water for injection, 0.3% lidocaine hydrochloride, 2.7% phosphate buffer, and 0.9% sodium chloride. |
Microspores provide permanent volume for wrinkle correction. |
FDA approved for correction of nasolabial folds. Lip volumizing contraindicated. |
Aseptic product that has an opaque, off-white appearance and is supplied in a sealed tray containing 5 syringes (3 with 0.8 mL, 2 with 0.4 mL); must be brought to room temperature prior to use; 26-gauge needle is used; best cosmetic result achieved by moving needle back and forth 2-3 times beneath each skin fold being treated, while maintaining constant pressure throughout implantation procedure; do not overcorrect because result is considered permanent. |
Permanent support structure for wrinkle correction. |
Safety of injecting more than 3.5 mL per treatment site or 8.9 mL overall not established. |
Poly-L-Lactic Acid
This novel product (Sculptra) differs from all other agents in several aspects. Poly-L-lactic acid is a synthetic, biodegradable, biocompatible, immunologically inert peptide polymer that is believed to stimulate fibroblasts to produce more collagen, thus increasing facial volume. In the United States, poly-L-lactic acid is only FDA approved for the treatment of HIV-associated lipoatrophy; and has since been approved for the correction of shallow to deep nasolabial fold (smile lines) contour deficiencies and other facial wrinkles in healthy patients. [24]
Although poly-L-lactic acid is nearly always injected subdermally, dermal neocollagenesis occurs, thus it is a dermal stimulating agent, not a true dermal filling agent. Several limitations have prevented poly-L-lactic acid from becoming as popular as other products. Poly-L-lactic acid must be premixed prior to use, making immediate treatment impossible. Unlike dermal fillers, results are not appreciated for 4 or more weeks. Lastly, most patients require 2-3 treatment sessions that are at least 4-6 weeks apart. [25]
Precautions
Because poly-L-lactic acid is not a true filler, but relies on neocollagenesis to achieve clinical improvement, the clinical results from this agent are less predictable than the true dermal fillers. Patients should be properly educated that results take 4-6 weeks to be appreciated. Dermal nodules have been reported after treatment and often take 7 months or much longer to develop. [1] When treating the face, these nodules can often be felt, but not seen. Reconstitution of the product with 6 or more milliliters of sterile water, in addition to vigorous posttreatment massage, is believed to reduce the incidence of nodule formation.
Use of this product on the hands has increased; however, unfortunately, the incidence of nodules in this location may be as high as 10% and the nodules are often visible, unsightly, and difficult to treat. In this location, treatment with Sculptra reconstituted with as much as 10 mL of sterile water has still resulted in nodule formation, which may take 1-3 years to appear after treatment. [26] Additional long-term studies are needed to fully assess the safety of poly-L-lactic acid for the treatment of the hands in an immune-competent individual.
No skin test is required prior to treatment. The product is stored at room temperature, although it must be reconstituted prior to treatment.
Table 8. Poly-L-Lactic Acid (Open Table in a new window)
Agent |
Contents |
Method of Action |
Indications |
Injection |
Duration |
Limits |
Sculptra |
Synthetic, biodegradable, biocompatible, immunologically inert polymer from the alpha-hydroxy-acid family; must be reconstituted with at least 3-5 mL of sterile water for injection; must stand for at least 2 hours to ensure hydration prior to treatment. |
Particles of poly-L-lactic acid stimulate formation of new collagen (collagen neosynthesis) in the skin, adding volume over time. |
Intended for restoration and/or correction of the signs of facial fat loss (lipoatrophy) in people with HIV infection; in immune-competent people, used as a single regimen for correction of shallow-to-deep nasolabial fold contour deficiencies and other facial wrinkles in which deep dermal grid pattern (cross-hatch) injection technique is appropriate. |
Supplied as a sterile, freeze-dried preparation for injection in a clear glass vial; to be injected into the deep dermis or subcutaneous layer. |
Approximately 1 year. |
Volume should be limited to approximately 0.1-0.2 mL per each individual injection; the volume of product injected per treatment area varies depending on surface area to be treated. |
Calcium Hydroxylapatite
This novel filler, Radiesse, was FDA approved in December 2006 for the correction of facial wrinkles and folds and for the correction of HIV-associated facial atrophy. In 2009, it received FDA approval for cosmetic use in non-HIV patients as well. The subdermal filler is composed of 30% calcium hydroxylapatite and 70% carrier gel. [27] The clinical results may last as long as 12 months or longer, although the carrier gel lasts no longer than 6 months, thus often resulting in a slight decrease in correction by that time.
Radiesse is nearly always injected subdermally at the dermal-subcutaneous junction or just above the periosteum; thus, this product is not a true dermal filler. Studies from 2008 suggest that calcium hydroxylapatite may induce neocollagenesis, although further research is needed. [28] Most commonly, Radiesse is used for the correction of nasolabial folds, atrophic cheeks, and temporal wasting.
Because of the pain associated with injection, some practitioners added lidocaine to the syringe, without a clinical appreciable decrease in effect. Mariano Busso, MD, [29] had treated numerous patients by adding 1 drop of 10% lidocaine to the previously available 1.3-mL syringe of Radiesse to decrease the discomfort associated with injection. In 2010, Radiesse with lidocaine was released to reduce pain upon injection and no premixing is needed.
Treatment of the hands has been accomplished with the addition of 0.15-0.23 mL of 2% lidocaine per 1.3-mL syringe of Radiesse. This can be accomplished using a nose-to-nose (female-to-female) Luer-lock connector to connect the syringe of Radiesse to a 3-mL syringe containing the lidocaine. At least 10 passes of the product back and forth between the two syringes is recommended to achieve adequate and even distribution of the lidocaine. This makes the consistency of the Radiesse slightly thinner, thus making it easier to spread when using a bolus injection technique. Similarly, this mixture is often preferred for the treatment of the temples.
Precautions
Injection into the dermis may result in nodule formation and should be avoided. Extreme care must be taken to avoid injection while withdrawing the needle out of the skin, which will result in the deposition of material into the dermis. Treatment of the lips has resulted in cyst formation containing the carrier gel. [21] For this reason, most cosmetic surgeons avoid treating the lips with Radiesse. Radiopaque particles of hydroxylapatite are visible on CT scans and radiolgraphy. Patients should be informed of this so they may inform their other healthcare providers.
No skin test is required prior to treatment, and the product is stored at room temperature.
Table 9. Synthetic Calcium Hydroxylapatite (Open Table in a new window)
Agent |
Contents |
Method of Action |
Indications |
Injection |
Duration |
Limits |
Radiesse |
Sterile, nonpyrogenic, semisolid, cohesive implant whose principal component is synthetic calcium hydroxylapatite suspended in a gel carrier of sterile water for injection, glycerin, and sodium carboxymethyl- cellulose; Radiesse (1.5 mL, 1.3 ML, 0.8 mL, and 0.3mL ) has a calcium hydroxylapatite particle size range of 25-45 μ m and should be injected with a 25- to 27-gauge needle. |
Stimulates formation of new collagen (collagenesis) in the skin, adding volume over time. |
Subdermal implantation for restoration or correction of signs of facial fat loss (lipoatrophy) in people with HIV infection; also for subdermal implantation for correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds. It is also used for correcting volume loss in the back of the hands and to restore the jawline. Treatment in adults over 21 years. |
Insert needle with bevel down at approximately 30° angle to skin; needle should slide under the dermis to the point where the injection should begin; advance the needle into the subdermis to the starting location; slowly inject material in linear threads, while withdrawing needle, until desired level of correction is achieved. |
Approximately 1 year, although the gel carrier is lost by 6 months, causing depreciation of initial results. |
Amount injected varies depending on site and extent of restoration or augmentation desired. Use a 1:1 correction factor. No overcorrection needed. |
| Radiesse (+) | Sterile, nonpyrogenic, semisolid, cohesive implant whose principal component is synthetic calcium hydroxylapatite suspended in a gel carrier of sterile water for injection, glycerin, and sodium carboxymethyl- cellulose; Radiesse (1.5 mL, 1.3 ML, 0.8 mL, and 0.3mL ) has a calcium hydroxylapatite particle size range of 25-45 μ m and should be injected with a 25- to 27-gauge needle. This product contains lidocaine to reduce pain upon injection. |
Stimulates formation of new collagen (collagenesis) in the skin, adding volume over time. | It is used for smoothing moderate to severe facial wrinkles and folds, such as nasolabial folds.. RADIESSE® is also used for correcting volume loss in the back of the hands and to restore the jawline. Treatment in adults over 21 years. | Insert needle with bevel down at approximately 30° angle to skin; needle should slide under the dermis to the point where the injection should begin; advance the needle into the subdermis to the starting location; slowly inject material in linear threads, while withdrawing needle, until desired level of correction is achieved | Approximately 1 year, although the gel carrier is lost by 6 months, causing depreciation of initial results | Amount injected varies depending on site and extent of restoration or augmentation desired. Use a 1:1 correction factor. No overcorrection needed. |
Autologous Cell Therapy
Azficel-T (laViv) is an autologous aesthetic cell therapy indicated to improve the appearance of moderate-to-severe nasolabial fold wrinkles in adults. The product is available from Fibrocell Science, Inc, a company focused on developing personalized cell therapies for aesthetic, medical, and scientific applications.
According to the company, creating azficel-T involves a patented technology whereby fibroblasts are extracted from behind the patient's ear and sent to the Fibrocell Science laboratory, where they are multiplied for about 3 months and are then frozen until needed. Only physicians who complete a Fibrocell-approved training program will be able to administer it.
This is a biological that works over time that provides gradual and natural results.
The FDA approval was based largely on two identical phase 3 multicenter, randomized, double-blind, placebo-controlled studies involving 421 patients who underwent 3 treatment sessions approximately 5 weeks apart. [30]
On the basis of investigators' and patients' assessments, a significantly greater proportion of patients demonstrated a positive response to treatment with azficel-T than with placebo. The treatment improved the appearance of nasolabial fold wrinkles for the 6 months of patient follow-up after the last treatment. The company said studies are ongoing, looking at how long beyond 6 months after the last treatment the effect may last.
In clinical trials, the most common adverse events were mild-to-moderate injection-site reactions that usually resolved within 1 week.
Table 10. Autologous Cell Therapy (Open Table in a new window)
Agent |
Contents |
Method of Action |
Indications |
Injection |
Duration |
Limits |
azficel-T (laViv) |
Cultured fibroblast cells obtained autologously are confirmed to contain collagen and suspended to form injectable biological as dermal filler. |
Following injection, the cultured fibroblasts are thought to synthesize new extracellular matrix and/or to stimulate remodeling of existing tissue components, thereby altering the structure, texture and appearance of the skin at injection site. |
Indicated to improve the appearance of moderate to severe nasolabial fold wrinkles. |
Manufacturing process for takes approximately 11-22 weeks after receipt of the patient’s biopsy samples by the manufacturer; each vial contains ~18 million autologous fibroblasts/1.2 mL. |
Six months, but possibly longer. |
Recommended regimen consists of a series of 3 intradermal injection sessions administered 3-6 weeks apart Inject 0.1 mL ID per linear centimeter into the nasolabial fold wrinkles. |
Summary
Considerable advances in technology have given the cosmetic surgeon an array of filler options. Still, no one filler can address every patient’s concern. Treatment should be tailored to the patient’s individual needs to achieve maximal benefit, minimize risk, and achieve the desired correction longevity. [31]
During the initial consultation, address the patient’s desires in detail, which should include the exact location of desired correction, duration of correction desired, expected degree of correction with treatment, expected duration of correction, and the potential adverse effects of treatment. Imperatively, communicate the limitations of treatment, including the degree of correction and the longevity, with the patient prior to treatment. Additionally, some patient’s will request that the physician use less dermal filler than is recommended due to cost. In this circumstance, fully communicate the reduction of the degree of improvement that will be seen prior to treatment, because this frequently results in an unsatisfied patient.
As with any cosmetic procedure, receiving informed consent and having effective communication with the patient prior to treatment is paramount.
Caution
In May 2015, the FDA issued a warning to healthcare providers and the public about serious complications that can occur if dermal fillers are inadvertently injected into blood vessels in the face. [2] The complications could possibly include vision impairment, blindness, stroke, and damage and/or necrosis of the skin and underlying facial structures. Caution should be used to ensure proper placement of the filler material, and patients should be informed about the potential adverse effects and how to recognize symptoms of impending serious complications.
Two separate but related reports from 2017 and 2018 have reaffirmed the risks, some very serious—including strokes and blindness—that can be associated with dermal fillers. [32] A 3-year study (2014-2016) reported swelling, infection, blindness (8 cases), and tissue death as the most common problems. A 10-year analysis (2007-2017) reported nodule formation, infection, inflammation, allergic complications, and vascular complications as the most common problems.
-
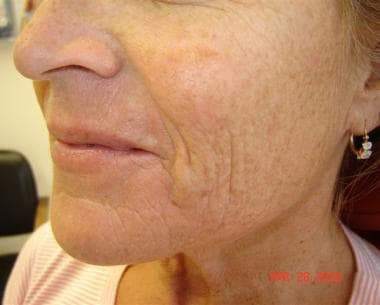
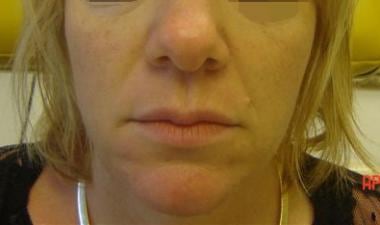
Patient 1. Before photo.
-
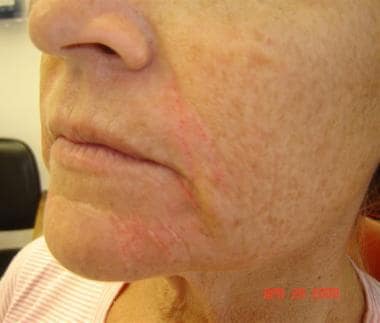
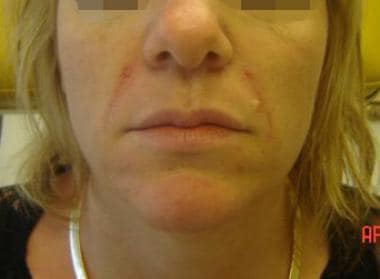
Patient 1. After photo. Dr. Bader injected CosmoDerm I (1 mL) into the nasolabial folds, oral commissure, and chin.
-
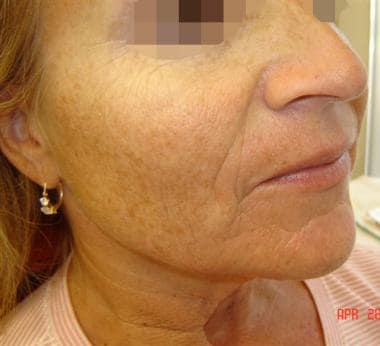
Patient 1. Before photo. Left cheek.
-
Patient 1. After photo. Left cheek.
-
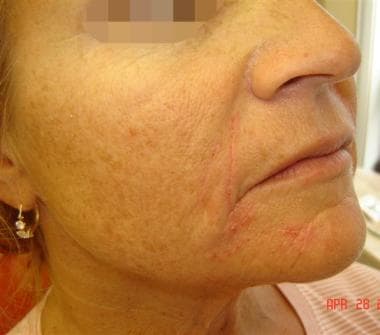
Patient 1. Before photo. Right cheek.
-
Patient 1. After photo. Right cheek.
-
Patient 2. Before photo.
-
Patient 2. After photo. Dr. Bader injected Restylane (1 mL) into the nasolabial folds.
-
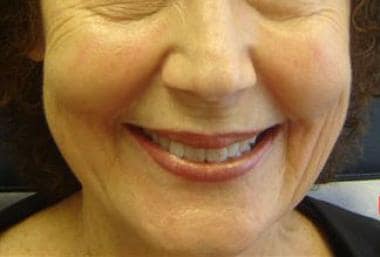
Patient 3. Before photo.
-
Patient 3. Before photo with smile to accentuate the areas of correction.
-
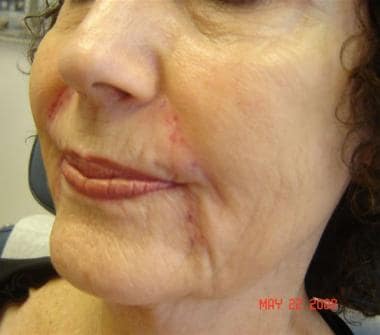
Patient 3. After photo. Dr. Bader injected CosmoDerm I (1 mL) into the glabella, lips, and crow's feet.
-
Patient 4. Before photo.
-
Patient 4. After photo. Dr. Bader injected Restylane (0.4 mL) into the nasolabial folds, chin, and oral commissure.
-
Patient 5. Before photo.
-
Patient 5. After photo. Dr. Bader injected Restylane (1 mL) into the nasolabial folds, chin, and oral commissure.
-
Patient 6. Before photo.
-
Patient 6. Dr. Bader injected CosmoDerm I (1 mL) into the nasolabial folds, upper and lower lips, left cheek, and lateral crow's feet (bilaterally).
-
Patient 7. Before photo.
-
Patient 7. After photo. Dr. Bader injected Perlane (1 mL) into nasolabial folds, upper and lower lips, and marionette lines.
-
Patient 7. After photo. Side view.