Practice Essentials
Pemphigus is an autoimmune bullous disease characterized by blisters and erosions of the skin and mucous membranes. Several variants of the disease exist, including pemphigus vulgaris, pemphigus foliaceous, and drug-induced pemphigus. Patients with drug-induced pemphigus have autoantibodies that are either circulating or tissue bound. [1] Since the 1950s, evidence has grown that drugs may cause or exacerbate pemphigus. A drug origin should be considered in every new patient with pemphigus. The most common variant of pemphigus associated with drug exposure is pemphigus foliaceus, although pemphigus vulgaris has also been described. In penicillamine-treated patients, pemphigus foliaceus is more common than pemphigus vulgaris, with an approximate ratio of 4:1.
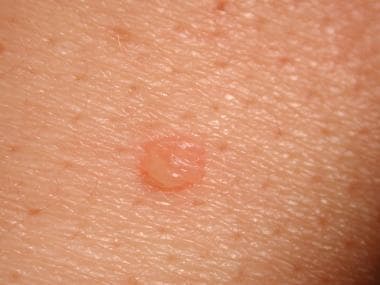
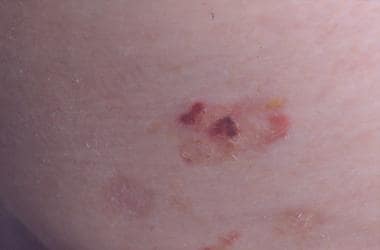
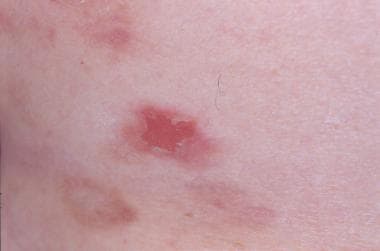
Note the images below.
History
Most patients develop the eruption a few weeks after starting therapy with the offending agent. In penicillamine use, the eruption may not develop until 6 months after the onset of therapy. Some patients may give a history of a nonspecific eruption prior to the development of pemphigus type lesions.
Complications
Secondary infections may occur in drug-induced pemphigus because of the disruption of the skin barrier. Extensive erosions may promote entrance of bacteria, resulting in cutaneous infections, bacteremia, or sepsis.
Treatment
See Medical Care and Medication.
Consultations
For patients who have erosions involving a significant portion of the body surface area, the burn unit is helpful in providing wound care (cleansing, application of topical antibiotics, and bandaging).
Patient education
Educate patients about their disease and their medications, including adverse effects from therapy.
The International Pemphigus and Pemphigoid Foundation, a nonprofit support group for patients with pemphigus and their families, offers an active web site and a quarterly newsletter, as well as local chapters in many parts of the country.
Pathophysiology
A variety of drugs have been implicated in the onset of drug-induced pemphigus. Some of these drugs induce antibody formation, which results in acantholysis via a mechanism identical to that found in idiopathic pemphigus. Other drugs are postulated to induce acantholysis directly in the absence of antibody formation. [2, 3]
Drugs that induce pemphigus may be categorized into 2 groups: thiol drugs and nonthiol drugs. Thiol drugs are reported most frequently as the culprits of drug-induced pemphigus. [4] They contain a thiol group (-SH) in their chemical structure. Penicillamine, captopril, and enalapril are the thiol drugs most often associated with drug-induced pemphigus. [5, 6]
Thiol drugs are postulated to induce acantholysis through biochemical mechanisms without antibody formation. Experiments with skin explants have demonstrated that thiol drugs can induce acantholysis directly. These investigations have resulted in several hypotheses regarding thiol-induced acantholysis, including the following:
-
Thiol drugs may interfere with critical enzymes, such as keratinocyte transglutaminase, resulting in loss of epidermal cell cohesion.
-
Thiol drugs may activate endogenous proteolytic enzymes, such as plasminogen activators, with subsequent cleavage of desmosomal antigens.
-
Thiol drugs may bind desmoglein 1 or desmoglein 3, creating a neoantigen, which then elicits an immune response.
-
Binding of the pemphigus antigens by thiol drugs may interfere with their normal function, resulting in acantholysis.
Nonthiol drugs include sulfur-containing drugs and drugs without sulfur in their structure. Sulfur-containing drugs, such as penicillins, cephalosporins, antihypertensive agents, and piroxicam, may undergo hydrolytic breakdown in vivo to form thiols; therefore, they are termed masked thiols. [4] An active amide group is found in the structure of many nonthiol drugs, which has resulted in the speculation that this structure may be responsible for the induction of disease. [7]
Nonthiol drugs are more likely to induce acantholysis via immune mechanisms. Studies of cases of non-thiol–induced pemphigus reveal the presence of autoantibodies that recognize pemphigus antigens, in particular desmoglein 3, which is the pemphigus vulgaris antigen. In fact, this group of patients tends to have clinical, histologic, immunologic, and prognostic features similar to idiopathic pemphigus vulgaris. [8]
One case report describes localized pemphigus foliaceus induced by topical imiquimod treatment. Imiquimod does not contain thiol, sulfur, or amide groups in its structure. The exact mechanism of acantholysis induction from this medication is unknown. Because imiquimod is known to cause a localized immune response at the site of application, the generation of antibodies to desmoglein 1 has been postulated as a mechanism of action.
Epidemiology
The estimated incidence of pemphigus is 1 in 100,000 people. [9] More than 200 cases of drug-induced pemphigus have been reported, with penicillamine accounting for almost 50%. In patients who take penicillamine for longer than 6 months, it is estimated that 7% develop pemphigus.
Most case series in the literature have not reported the race of patients with drug-induced pemphigus. A number of reports from Israel of drug-induced pemphigus occurring in Jewish persons of Ashkenazi origin suggest an ethnic predominance.
A study evaluating the epidemiology of pemphigus in the Mediterranean region of Turkey found a female predominance (male-to-female ratio, 1:1.4).
Drug-induced pemphigus can occur at any age. In reported cases, patient age has ranged from the third to ninth decade.
Prognosis
Patients with thiol-induced pemphigus and patients lacking cell surface autoantibodies have a more favorable prognosis. Up to 50% of thiol-induced pemphigus cases remit upon withdrawal of the drug.
Patients with pemphigus induced by nonthiol drugs are more likely to have cell surface antibodies and to have a chronic course similar to idiopathic pemphigus vulgaris.
Mortality rates for drug-induced pemphigus have not been published. A fatal case of acute onset pemphigus vulgaris has been reported in a patient treated with interferon beta and recombinant interleukin 2. [10]
Significant morbidity may occur. Patients with extensive cutaneous lesions report significant pain and burning sensations. Oral involvement also causes significant pain and results in decreased oral intake. This may result in dehydration.
-
Early small blister filled with clear fluid arises on healthy skin.
-
Flaccid blister filled with clear fluid arises on healthy skin.
-
An erosion.