Practice Essentials
Reactive arthritis (ReA), formerly termed Reiter syndrome, is an autoimmune condition that develops in response to an infection. It has been associated with gastrointestinal (GI) infections with Shigella, Salmonella, Campylobacter, and other organisms, as well as with genitourinary (GU) infections (especially with Chlamydia trachomatis). [1] Cases of ReA following COVID-19 infection have been reported. [2, 3, 4, 5, 6]
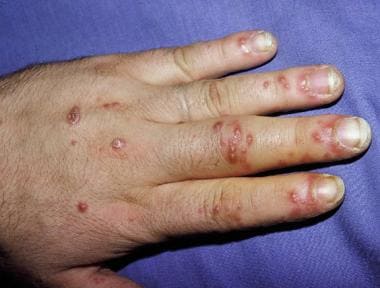
See the image below.
Signs and symptoms
The classic triad of ReA symptoms (found in only one third of patients) consists of the following:
-
Noninfectious urethritis
-
Arthritis
-
Conjunctivitis
In postenteric ReA, diarrhea and dysenteric syndrome (usually mild) is commonly followed by the clinical triad in 1-4 weeks. Some add a fourth component (mucocutaneous findings) to make up a diagnostic tetrad.
The following may be noted:
-
Acute onset of ReA, with malaise, fatigue, and fever
-
Asymmetrical, predominantly lower-extremity, oligoarthritis as the major presenting symptom, sometimes with early myalgias
-
Initial nongonococcal urethritis, with frequency, dysuria, urgency, and urethral discharge
-
In addition to conjunctivitis, ophthalmologic symptoms that include erythema, burning, tearing, photophobia, pain, and decreased vision (rare)
-
Mild recurrent abdominal complaints after a precipitating episode of diarrhea
-
In HIV-positive patients, severe psoriasiform dermatitis, commonly involving the flexures, scalp, palms, and soles
Physical findings in ReA may include the following:
-
Musculoskeletal system - Asymmetric oligoarthritis affecting the weight-bearing joints, predominantly of the lower extremities; sausage-shaped finger (dactylitis); enthesopathy; sacroiliitis
-
Skin and nails - Keratoderma blennorrhagicum; erythema nodosum (uncommon); onychodystrophy
-
Eyes - Conjunctivitis; anterior uveitis; keratitis; scleritis; episcleritis; cataracts; hypotony; glaucoma; corneal ulceration; disc or retinal edema; retinal vasculitis; optic neuritis; dacryoadenitis
-
GU tract - Meatal edema and erythema and clear mucoid discharge; prostatitis; vulvovaginitis; circinate balanitis (balanitis circinata); cervicitis; cystitis; salpingo-oophoritis; pyelonephritis; bartholinitis
-
GI tract - Diarrhea; abdominal pain; lesions resembling inflammatory bowel disease on ileocolonoscopy
-
Other systems - Cardiac (aortitis, aortic regurgitation, transient conduction abnormalities, myocarditis, pericarditis); renal (proteinuria, microhematuria, amyloid deposits, immunoglobulin A [IgA] nephropathy)
See Presentation for more detail.
Diagnosis
The diagnosis of ReA is clinical, based on the history and physical examination. No laboratory study or imaging finding is diagnostic.
The following laboratory studies may be helpful:
-
White blood cell (WBC) and red blood cell (RBC) counts
-
Erythrocyte sedimentation rate (ESR)
-
C-reactive protein (CRP) and other acute-phase reactants
-
IgA antibodies to specific bacterial antigens
-
Serology and cultures (blood, urine, stool, cervix, urethra), particularly for Chlamydia
-
Human leukocyte antigen (HLA)–B27
-
Tuberculin skin test
-
HIV
-
Urinalysis
Imaging modalities that may be considered include the following:
-
Plain radiography (reveals findings in only 40-70% of cases)
-
Whole-body scintigraphy
-
Positron emission tomography
-
Magnetic resonance imaging (MRI), especially of the sacroiliac joints
-
Computed tomography (CT)
-
Ultrasonography or echocardiography
Other studies to be considered include the following:
-
Arthrocentesis, synovial fluid analysis, and synovial biopsy (often needed to rule out an infectious process, especially in monoarticular arthritis with constitutional symptoms)
-
Antistreptolysin O (ASO) or anti-DNase B testing (if poststreptococcal infection is suspected)
-
Electrocardiography (ECG; in patients with a prolonged course of ReA)
See Workup for more detail.
Management
No curative treatment exists; instead, treatment aims at relieving symptoms and is based on symptom severity. Almost two thirds of patients have a self-limited course; as many as 30% develop chronic symptoms, posing a therapeutic challenge.
Pharmacologic agents that may be used in treating ReA include the following:
-
Nonsteroidal anti-inflammatory drugs (NSAIDs) - These are the mainstays of therapy
-
Corticosteroids (topical, intra-articular, and systemic)
-
Antibiotics (for Chlamydia-related ReA in particular) - Tetracyclines are commonly given
-
Disease-modifying antirheumatic drugs (DMARDs) - Typically given when NSAIDs are ineffective or contraindicated, these include sulfasalazine, methotrexate, and anti−tumor necrosis factor (TNF) medications such as etanercept and infliximab
No specific surgical treatment is indicated, though ophthalmologic surgery may be warranted to treat certain ocular manifestations of disease.
Physical therapy may be instituted to avoid muscle wasting and to reduce pain.
See Treatment and Medication for more detail.
Background
Reactive arthritis (ReA) is an autoimmune condition that develops in response to an infection. [7, 8] ReA has been associated with gastrointestinal (GI) infections with Shigella, Salmonella, Campylobacter, and other organisms, as well as with genitourinary (GU) infections (especially with Chlamydia trachomatis).
ReA was described by the German physician Hans Reiter in 1916, [9] and for a time the disorder was known as Reiter syndrome. This eponym is no longer used, because of Reiter’s activities as a Nazi war criminal, and also because his was not the first description of ReA, and it mischaracterized the pathogenesis. [10, 11]
As currently understood, the term ReA encompasses the older concepts of complete and incomplete reactive arthritis and a clinical syndrome of arthritis with or without extra-articular features that develop within 1 month of infectious diarrhea or GU infection. The classic triad associated with this condition comprises noninfectious urethritis, arthritis, and conjunctivitis (though this triad is not found in all cases).
ReA is frequently associated with the human leukocyte antigen (HLA)–B27 (HLA-B27) haplotype and is classified in the category of seronegative spondyloarthropathies, which includes ankylosing spondylitis, psoriatic arthritis, the arthropathy of associated inflammatory bowel disease, juvenile-onset ankylosing spondylitis, juvenile chronic arthritis, and undifferentiated spondyloarthritis. [12]
A study by Kaarela et al reported that ReA and ankylosing spondylitis appear to be identical. [13] When assessing long-term outcomes of reactive arthritis and ankylosing spondylitis to identify similarities in manifestations of disease, the investigators found a number of similarities. Among these was the determination that sacroiliitis, peripheral arthritis, and iritis developed most often in both chronic ReA and ankylosing spondylitis.
Most ReA patients are young men. Young children tend to have the postdysenteric form, whereas adolescents and young men are most likely to develop ReA after a genitourinary infection. Some authors, interpreting the mucocutaneous findings as pustular psoriasis and the seronegative arthritis as psoriatic arthritis, believe that ReA is best classified as a type of psoriasis. [14]
Defining criteria
The classic triad of ReA—namely, arthritis, conjunctivitis, and noninfectious urethritis—occurs in only about one third of patients at onset. Less stringent diagnostic criteria from the American College of Rheumatology have specified a 1-month duration of arthritis in association with urethritis, cervicitis, or both.
It has been suggested, however, that the syndrome is better described as a triad consisting of arthritis, conjunctivitis or iridocyclitis, and nonbacterial urethritis or cervicitis. Some prefer to describe it as a tetrad, adding the mucocutaneous findings of balanitis circinata and keratoderma blennorrhagicum to the classic triad. In this view, the complete and incomplete forms of ReA can be identified by the presence or absence of the mucocutaneous involvement.
Pathophysiology
ReA is usually triggered by a GU or GI infection (see Etiology). Evidence indicates that a preceding Chlamydia respiratory infection may also trigger ReA. [15] The frequency of ReA after enteric infection averages 1-4% but varies greatly, even among outbreaks of the same organism. Although severely symptomatic GI infections are associated with an increased risk of ReA, [16, 17] asymptomatic venereal infections more frequently cause this disease. [16] About 10% of patients have no preceding symptomatic infection.
ReA is associated with histocompatibility leukocyte antigen B-27 (HLA-B27), a major histocompatibility complex (MHC) class I molecule involved in T-cell antigen presentation. Results for HLA-B27 are positive in 65-96% of patients (average, 75%) with ReA. [18] Patients with HLA-B27, as well as those with a strong family clustering of the disease, tend to develop more severe and long-term disease. [13]
Sun et al reported that susceptibility to ReA arthritis is affected by the levels of certain killer cell immunoglobulin-like receptors (KIRs), which correspond with specific HLA-C ligand genotypes. In individuals with high levels of activating and low levels of inhibitory KIR signals, pathogens can more easily trigger natural killer cell and T cell innate and adaptive immune responses, resulting in the overproduction of cytokines that contribute to the pathogenesis of ReA. [19]
Their study of 138 patients with ReA found that KIR2DS1, which is activating, is associated with susceptibility to ReA, when present alone or in combination with the HLA-C1C1 genotype. KIR2DL2, which is inhibitory, in combination with the HLA-C1 ligand is associated with protection against ReA. Patients with ReA had significantly lower frequencies of KIR2DL2 and KIR2DL5 than did controls. The presence of more than seven inhibitory KIR genes was protective. [19]
The mechanism by which the interaction of the inciting organism with the host leads to the development of ReA is not known. It is possible that microbial antigens cross-react with self-proteins, stimulating and perpetuating an autoimmune response mediated by type 2 T helper (Th2) cells. Chronicity and joint damage have been associated with a Th2 cytokine profile that leads to decreased bacterial clearance. [16]
Synovial fluid cultures are negative for enteric organisms or Chlamydia species. However, a systemic and intrasynovial immune response to the organisms has been found with intra-articular antibody and bacterial reactive T cells. Furthermore, bacterial antigen has been found in the joints. Thus, the elements for an immune-mediated synovitis are present.
Synovitis in ReA is mediated by proinflammatory cytokines. Native T cells under the influence of transforming growth factor (TGF)-β and other cytokines, such as interleukin (IL)-6, differentiate into Th17 effector cells, which then produce IL-17. IL-17 is one of the major cytokines elevated in the synovial fluid of these patients. [20, 21] Deficiencies in regulatory mechanisms can result in increased proinflammatory cytokine production and worse outcome. [22]
The Toll-like receptors (TLRs) recognize different extracellular antigens as part of the innate immune system. [23] TLR-4 recognizes gram-negative lipopolysaccharide (LPS). Studies in mice and humans showed abnormalities in antigen presentation due to downregulation of TLR-4 costimulatory receptors in patients with ReA. Subsequent studies implicated TLR-2 polymorphism associated with acute ReA; however, its role is still disputed. [16, 24]
Molecular evidence of bacterial DNA (obtained via polymerase chain reaction [PCR] assay) in synovial fluids has been found only in Chlamydia -related ReA, and a single placebo-controlled trial of a tetracycline derivative (ie, lymecycline) has shown a reduction in the duration of acute Chlamydia -related, but not enteric-related, ReA. [25] This suggests that persistent infection may play a role, at least in some cases of chlamydial-associated ReA.
In a subsequent trial, the combination of doxycycline and rifampin was superior to doxycycline alone in reducing morning stiffness and swollen and tender joints in patients with undifferentiated spondyloarthropathy. [26]
The role of HLA-B27 in this scenario remains to be fully defined. The following theories have been proposed:
-
Molecular mimicry - This hypothesis suggests that a similarity exists at the molecular level between the HLA-B27 molecule and the inciting organisms, allowing the triggering of an immune response and the subsequent development of clinical disease [27]
-
HLA-B27 as a receptor for certain bacteria - At present, there is little evidence either to confirm or to refute this hypothesis
-
Defective class I antigen-mediated cellular response - This hypothesis suggests that the HLA-B27 molecule may be a defective molecule associated with an aberrant cytotoxic T-cell response
ReA can occur in patients with HIV infection or AIDS—most likely because both conditions can be sexually acquired, rather than because ReA is triggered by HIV. The course of ReA in these patients tends to be severe, with a generalized rash resembling psoriasis, profound arthritis, and frank AIDS. HLA-B27 frequency is the same as that associated with non–AIDS-related ReA in a similar demographic group. This association points out the likely importance of CD8+ cytotoxic T cells as compared with CD4+ Th cells in the pathogenesis of ReA.
ReA is sometimes divided into epidemic and endemic forms. Whereas a triggering agent can be identified for epidemic ReA, none has been identified for endemic ReA. Differentiation between the 2 types of ReA may be difficult in some cases; however, it is not essential to either diagnosis or treatment.
Etiology
Infectious causes
ReA is usually triggered by a GU or GI infection and thus is sometimes classified as venereal or dysenteric. Such infections are mostly the result of gram-negative, obligate, or facultative intracellular pathogens. [28] Organisms that have been associated with ReA include the following:
-
Ureaplasma urealyticum
-
Neisseria gonorrhoeae
-
Shigella flexneri
-
Mycoplasma pneumoniae
-
Mycobacterium tuberculosis [34]
-
Cyclospora [35]
-
Yersinia enterocolitica and Y pseudotuberculosis
-
Campylobacter jejuni [36] and C coli
-
Clostridioides difficile
-
Giardia lamblia [37]
-
Leptospira [38]
-
Babesia microti [38]
Data suggest that chlamydial ReA is underdiagnosed and that asymptomatic chlamydial infections might be a common cause. [40] An important difference between Chlamydia-induced (postvenereal) ReA and postenteric ReA is the presence of viable but aberrant chlamydial organisms in the synovial fluid [41] (so-called Chlamydia persistence [42] ). Polymerase chain reaction (PCR) assay to detect C trachomatis DNA in synovial samples may be a good method for establishing the diagnosis of Chlamydia-induced arthritis in patients with ReA. [43]
The prevalence of different serotypes of C trachomatis antibodies and the incidence of Chlamydia-induced ReA was studied in patients with early arthritis in a defined population in Finland. [18] Antibodies against C trachomatis were most common in patients with arthritis because cases with Chlamydia-induced ReA are included in this subgroup.
Ureaplasma organisms are known to be capable of causing experimental and clinical nongonococcal urethritis. Synovial mononuclear cells from arthritic joints of patients with ReA react with Ureaplasma antigens; this organism has been isolated from an ReA patient.
The enteric pathogen that most commonly results in ReA is Campylobacter (C jejuni, 90-95%; C coli, 5-10%). [44] ReA patients with arthritic symptoms are more frequently infected with C jejuni strains with sialic acid lipo-oligosaccharide. In addition, sialylation of C jejuni lipo-oligosaccharide is associated with more severe enteric disease. [45]
Group A streptococci are known to be capable of causing poststreptococcal ReA. [46] Patients with this condition demonstrate increase antistreptolysin O (ASO) antibodies and an increased erythrocyte sedimentation rate (ESR). [47] ReA can also be induced by tonsillitis. In one study, 13 of 21 patients were positive for ASO and 12 were positive for group A Streptococcus. [48]
Acute tuberculosis can sometimes cause ReA. The resulting condition is known as Poncet disease, which is a different entity from tuberculous arthritis. [49, 50, 51]
ReA following infection with C difficile has been reported. [52, 53] Intravesical instillation of bacillus Calmette-Guérin (BCG) for bladder cancer has been associated with ReA. [54, 55, 56] has also been shown to occur after tetanus and rabies vaccination. [57, 58]
Genetic factors
ReA has an important genetic component; it tends to cluster in certain families and almost exclusively affects males, and HLA-B27 is identified in 70-80% of patients. [18] HLA-B27 may share molecular characteristics with bacterial epitopes, facilitating an autoimmune cross-reaction instrumental in pathogenesis. HLA-B27 contributes to the pathogenesis of the disease and reportedly increases the risk of ReA 50-fold. [59] HLA-B51 and HLA-DRB1 alleles have also been shown to be associated with ReA. [43]
Rihl et al found a high proportion of proangiogenic factors accounting for a genetically determined susceptibility to ReA. [60] Sun et al reported increased susceptibility to ReA associated with the HLA-C1C1 genotype, which indicates the absence of the HLA ligands for the inhibitory killer cell immunoglobulin-like receptor (KIR) KIR2DL1; Imbalance between activating and inhibitory KIR signals may allow pathogens to trigger cytokine overproduction. [19]
Other factors
ReA triggered by adalimumab and leflunomide in a patient with ankylosing spondyloarthropathy and Crohn disease has been described. [61] Duration of diarrhea and weight loss are also considered risk factors in the development of ReA after enteric infections. [17]
Epidemiology
United States statistics
Data on the incidence and prevalence of ReA are scarce, partly because of a lack of a disease definition and classification criteria; these factors complicate differentiation of ReA from other arthritides. [62] The frequency is estimated to be 3.5-5 cases per 100,000. The incidence reported in US Navy personnel over a 10-year period was 4 cases per 100,000 men per year. The prevalence of ReA may be relatively high among patients with AIDS, especially men who are seropositive for HLA-B27. ReA develops in almost 75% of HIV-positive men with HLA-B27.
An estimated 1-3% of all patients with a nonspecific urethritis develop an episode of arthritis. The incidence is 1-4% after enteric infection. This number jumps to 20-25% after bacterial enteritis in HLA-B27-positive individuals. [44] Prevalence of asymptomatic chlamydial infections, underdiagnosis, and underreporting may make the incidence even higher. [63] It has been noted that worse functional capacity and higher disease activity are observed in the lower socioeconomic classes. [64]
A population-based study assessed ReA after culture-confirmed infections with bacterial enteric pathogens in Minnesota and Oregon. [65] The estimated incidence after culture-confirmed Campylobacter, Escherichia coli O157, Salmonella, Shigella, and Yersinia infections in Oregon was 0.6-3.1 cases per 100,000 population. ReA may occur in 1.5% of Shigella enterocolitis cases and 25% of HLA-B27–positive Shigella cases. After an outbreak of S enterica serovar Enteritidis, 29% had reactive arthritis. [32]
International statistics
The infections that incite ReA may vary with geographic location. For example, Y enterocolitica is more commonly identified in Europe than in North America and thus is responsible for more cases of ReA in countries such as Finland and Norway. [16] The occurrence of ReA appears to be related to the prevalence of HLA-B27 in a population and to the rate of urethritis/cervicitis and infectious diarrhea.
More than 40 subtypes of HLA-B27 are known; those associated with the spondyloarthropathies are HLA-B2702, B2704, and B2705. [28] These subtypes may be somewhat geographically segregated. For example, the subtype B2705 is found predominantly in Latin America, Brazil, Taiwan, and parts of India. It is noteworthy that subtypes HLA-B2706 and B2709—found in native Indonesia and Sardinia, respectively—may be partially protective against ReA. [66]
In Norway, an annual incidence of 4.6 cases per 100,000 population for chlamydial ReA and an incidence of 5 cases per 100,000 population for enteric bacteria–induced ReA were reported in 1988-1990. In Finland, nearly 2% of males were found to have ReA after nongonococcal urethritis; the incidence of HLA-B27 is higher in the Finnish population. In the United Kingdom, the incidence of ReA after urethritis is about 0.8%. In the Czech Republic, the annual incidence of ReA in adults during 2002-2003 was reported at 9.3 cases per 100,000 population. [67]
Age-, sex-, and race-related demographics
ReA is most common in young men, with the peak onset in the third decade of life. It rarely occurs in children; when it does, the enteric form of the disease is predominant. Most pediatric patients present with symptoms after the age of 9 years. [54]
In a study of 100 patients with ReA, Lahu and colleagues found that most patients were between 20 and 40 years old and that the first attack occurred earlier in males than females. Of the 100 patients studied, 66% were male. Urogenital and nasopharyngeal infections were more common among male patients. [68]
ReA after foodborne enteric infections is equally common in males and females. However, the male-to-female ratio for disease associated with venereally acquired infections has been estimated to range from 5:1 to 10:1. A possible prostatic focus of persistent infection is postulated to explain the male predominance of ReA.
The frequency of ReA appears to be related to the prevalence of HLA-B27 in the population. As with other spondyloarthropathies, HLA-B27 and ReA are more common in white people than in black people. When ReA occurs in black persons, it is frequently B27-negative.
Prognosis
ReA has a variable natural history [63] but typically follows a self-limited course, with resolution of symptoms by 3-12 months, even in patients who are acutely incapacitated. A fatal outcome is seldom reported, but death can occur, and it is usually related to the adverse effects of treatment. Postdysenteric cases are associated with a better prognosis than postvenereal cases. The presence of HLA-B27 may predict a more prolonged course and severe outcome, as may infections triggered by Yersinia, Salmonella, Shigella, or Chlamydia. [69]
ReA has a high tendency to recur (15-50% of cases), particularly in individuals who are HLA-B27–positive. A new infection or other stress factor could cause reactivation of the disease.
Approximately 15-30% of patients with ReA develop a long-term, sometimes destructive, arthritis or enthesitis or spondylitis. A 1994 study analyzed 7 factors as predictors of long-term outcome in spondyloarthropathies. [70] The number of patients with ReA in this study was low, and a valid subgroup analysis was impossible. The presence of hip-joint involvement, an ESR higher than 30, and unresponsiveness to nonsteroidal anti-inflammatory drugs (NSAIDs) probably portend a severe outcome or chronicity in ReA.
Patient Education
Poor health-related quality of life and impaired daily physical functioning are observed in patients with refractory or chronic ReA, and strategies focused on improving or maintaining functional status are important in treatment. [24] Educational measures that may be helpful include the following:
-
Encourage patients to monitor themselves for any changes in symptoms.
-
Help patients to become awareness of the chronic or recurrent nature of this syndrome and to accept the need for long-term medications
-
Inform patients that their condition places them at a higher than usual risk for elective ocular surgery
-
Discourage inactivity and immobilization
-
Encourage stretching exercises and range of motion
-
Provide adolescent patients with information on the prevention of sexually transmitted diseases and the use of condoms
For patient education resources, see the Arthritis Center, the Sexually Transmitted Diseases Center, and the Bacterial and Viral Infections Center, as well as Knee Pain, Chlamydia, and Gonorrhea. The American College of Rheumatology also provides patient information on reactive arthritis.
-
Balanitis circinata (circinate balanitis) in patient with reactive arthritis.
-
Plaques on soles of patient with reactive arthritis.
-
Painful erosions on fingers in patient with reactive arthritis.
-
Plaques and erosions of tongue in patient with reactive arthritis.
-
Radiograph of feet of 27-year-old man shows erosions in all left metatarsophalangeal (MTP) joints with subluxation and valgus deformity of most toes. Smaller erosions are also visible in fourth and fifth MTP joints of right foot.
-
Lateral radiograph of foot reveals calcaneal spur and enthesitis.
-
Radiograph of both hands shows small erosive changes in both first metacarpal heads associated with minimal subluxation. Bone density is normal.
-
Radiography of pelvis reveals bilateral asymmetric sacroiliitis.
-
Radiograph in 40-year-old man shows nonmarginal syndesmophytes predominantly in lower thoracic and upper lumbar spine.
-
Swelling of right knee with effusion caused by arthritis. Image courtesy of Gun Phongsamart, MD.
-
Remarkable tenderness of left sacroiliac joint caused by sacroiliitis. Image courtesy of Gun Phongsamart, MD.
-
Balanitis circinata (circinate balanitis). Image courtesy of Gun Phongsamart, MD.
-
Achilles tendinitis and swelling of retrocalcaneal bursa.