Practice Essentials
Lamellar ichthyosis (LI) is an autosomal recessive disorder that is apparent at birth and is present throughout life. The newborn is born encased in a collodion membrane that sheds within 10-14 days. The shedding of the membrane reveals generalized scaling with variable redness of the skin. The scaling may be fine or platelike, resembling fish skin. However, in at least 1 reported case, a premature neonate with LI was born without a collodion membrane. [1] Although the disorder is not life threatening, it is quite disfiguring and causes considerable psychological stress to affected patients. [2]
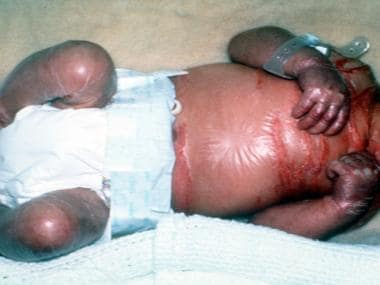
See the image below.
Along with harlequin ichthyosis and congenital ichthyosiform erythroderma, LI is classified as an autosomal recessive congenital (ARC) ichthyosis. [3]
Causes
Lamellar ichthyosis is an autosomal recessive disorder in almost all cases. As such, consanguinity is a major predisposing factor. Genetic linkage studies have been performed on families with classic lamellar ichthyosis and show markers on band 14q11 in the region of the TGM1 gene locus. An autosomal dominant form of lamellar ichthyosis has been described. [4, 5] Paller et al concluded that the major orphan forms of ichthyosis share an interleukin 17–dominant immune fingerprint. [6]
Complications
Patients with lamellar ichthyosis have a normal life expectancy. Scaling of the skin may lead to obstruction of sweat glands, resulting in thermoregulatory complications. Bacterial colonization in areas of excessive scaling may lead to chronic ear infection. Nail dystrophy, ectropion formation, and scarring alopecia are other complications of lamellar ichthyosis. [7]
Diagnostics
As a result of the abnormal skin barrier, neonatal sepsis is a significant risk in the newborn period. If sepsis is considered, perform a sepsis workup. Chemistries and fluids need to be monitored closely because of the high incidence of hypernatremia observed.
Skin biopsies can aid in the diagnosis of lamellar ichthyosis and detection of transglutaminase-1 expression. At birth, electron microscopy can be used to differentiate a severe collodion baby affected by lamellar ichthyosis from a baby affected by harlequin ichthyosis by demonstrating the absence of the marginal band. [8]
Also see Workup.
Treatment
See Treatment and Medication.
Pathophysiology
Patients with lamellar ichthyosis have accelerated epidermal turnover with proliferative hyperkeratosis, in contrast to retention hyperkeratosis. This involves a mutation in the gene for transglutaminase 1 (TGM1). There are at least 14 identified different TGM1 mutations. [9] The transglutaminase 1 enzyme is involved in the formation of the cornified cell envelope. The formation of the cornified cell envelope is an essential scaffold upon which normal intercellular lipid layer formation in the stratum corneum occurs. Thus, mutations in the TGM1 secondarily cause defects in the intercellular lipid layers in the stratum corneum, leading to defective barrier function of the stratum corneum and to the ichthyotic phenotype seen in lamellar ichthyosis patients and in transglutaminase 1 knockout mice. How much a defective cornified cell envelope alone contributes to the barrier abnormality in ichthyoses remains unclear. [10]
Compound heterozygous missense mutations p.Leu207Pro and p.Tyr544Cys in TGM1 have been identified as the causes of a particularly severe form of lamellar ichthyosis. [11] To date, several other genes for lamellar ichthyosis have been localized and identified, as follows [12] :
Epidemiology
Lamellar ichthyosis affects all populations, and the prevalence is less than 1 case per 300,000 individuals. Incidence in males and females is equal. The disease is present at birth and continues throughout life.
It should be noted that both autosomal recessive lamellar ichthyosis and X-linked recessive ichthyosis have been identified in a consanguineous family. [15]
A rare phenotype of lamellar ichthyosis has been described in South Africa. The term bathing-suit ichthyosis describes the characteristic distribution of the lesions, which involve the trunk, the proximal parts of the upper limbs, the scalp, and the neck, with sparing of the central face and extremities. This form of lamellar ichthyosis is caused by temperature sensitive and non-temperature sensitive mutations in TGM1. [16, 17, 18]
Prognosis
Patients with lamellar ichthyosis have normal life spans.
In the neonatal period, following the shedding of the collodion membrane, the newborn is at risk for secondary sepsis and hypernatremic dehydration.
As the child ages, the hyperkeratosis can interfere with normal sweat gland function, which can predispose to heat intolerance and possible heat shock. Ectropion may result in the inability to fully close the eyelids and can cause exposure keratitis.
External auditory canal stenosis and tympanic membrane blunting may result in a conductive hearing loss. Osseointegrated hearing devices may effectively bypass this hearing defect.
Less common associations include orthopedic abnormalities such as genu valgum, other ocular problems such as corneal perforation, and rickets. [19]
Like other ichthyoses, lamellar ichthyosis may be especially prone to widespread, severe, and chronic Trichophyton rubrum infections and viral infections. [12, 20, 21]
Patient Education
The family should be aware of these patient and family support groups:
-
Foundation for Ichthyosis and Related Skin Types (F.I.R.S.T), 2616 N. Broad Street, Colmar, PA 18915; telephone: (215) 997-9400, http://www.scalyskin.org, email: info@scalyskin.org.
-
National Registry for Ichthyosis and Related Disorders, University of Washington, Dermatology Department, Rm. BB1353, Box 356524, 1959 NE Pacific St., Seattle, WA 98195-6524; telephone: (206) 616-3179 or (800) 595-1265, email: info@skinregistry.org
-
Collodion baby with translucent membrane of the body.
-
Keratoderma of the palms in a patient with lamellar ichthyosis. Courtesy of Dirk Elston, MD.
-
Inflexible fingers due to taut skin in a young patient with lamellar ichthyosis. Courtesy of Dirk Elston, MD.
-
Nail dystrophy and inflammation of the nail folds. Courtesy of M. Bryan, MD.