Practice Essentials
Bullous or blistering drug eruptions and drug-induced anaphylaxis and hypersensitivity syndromes are among the most serious types of adverse drug reactions. Based on the various mechanisms, bullous drug eruptions may be classified into the following categories:
-
Spongiotic or eczematous
-
Acute generalized exanthematous pustulosis
-
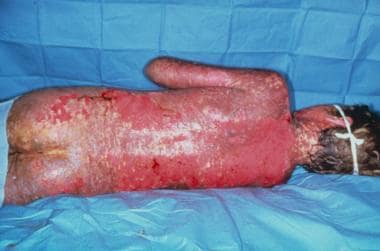
Erythema multiforme (EM), Stevens-Johnson syndrome (SJS) (see the image below), or toxic epidermal necrolysis (TEN)
-
Drug-induced pemphigoid
-
Drug-induced linear immunoglobulin A (IgA) dermatosis [1]
-
Pseudoporphyria cutanea tarda
Signs and symptoms
The physical findings of bullous drug eruptions vary greatly depending on the type of reaction.
On physical examination, the features of an eczematous drug eruption are similar to that of a diffuse contact dermatitis. These features include diffuse patches of erythema, microvesiculation, vesicles, crusts, and oozing. Other more specific features may include dyshidrotic hand dermatitis, EM-like lesions, purpura, urticarial lesions, and vasculitislike lesions. Recrudescence of a positive patch test reaction may occur after systemic exposure to the offending medication. A diffuse eczematous eruption may mimic severe atopic dermatitis.
See Presentation for a full discussion.
Diagnostics
Please see Workup.
Management
Withdrawal of the offending medication is the most important aspect of treatment of bullous drug reactions. Most reactions are self-limited. Conservative treatment of these disorders involves using wet compresses of Burrow solution and the application of moderate- to high-potency topical corticosteroids. More severe reactions may require the use of systemic corticosteroids.
The use of corticosteroids in the treatment of SJS and TEN is controversial. Patients with SJS and TEN are usually managed as inpatients in the intensive care or burn units. Fluid hydration, electrolyte balance, and nutritional support are the cornerstones of therapy. Rigorously guard against infection. Intravenous gamma globulin (IVIG) shows promise in the treatment of TEN. The IVIG reduces apoptosis by blocking CD95 on T cells. [2] In TEN, early withdrawal of precipitating drugs may reduce mortality if the drug has a short half-life. [3]
Limited forms of EM can be managed on an outpatient basis; however, careful consideration should be given to patients with SJS and TEN regarding an early referral to an intensive care unit or preferably a burn unit. Eye involvement that can occur in EM, SJS, and TEN requires an ophthalmologic evaluation.
There is a reported case of a woman with drug-induced bullous pemphigus secondary to head and neck cancer treatment with an immunoglobulin-like transcript 4 inhibitor (MK-4830) combined with pembrolizumab. She was treated with upadacitinib, a Janus-associated kinase-1 (JAK-1 inhibitor with good response at 4 weeks followup. [4]
Treatment with dipeptidyl peptidase 4 (DPP4) inhibitors is a common etiology of drug-induced bullous pemphigus. A literature review suggested that simultaneous use of the angiotensin-converting enzyme (ACE) inhibitor, lisinopril, can prevent bullous pemphigus in these patients. [5]
In an interesting revelation, there have been at least 17 document cases of drug-induced bullous pemphigus both caused and treated by the same biologics. The specific biologics were anti-tumor necrosis factor (aTNF)-α therapies, interleukin (IL)-17 inhibitors, and IL-12/IL-23 or IL-23 inhibitors. [6]
Pathophysiology
As with other bullous disorders, drug-induced blistering reactions occur via a variety of pathophysiological mechanisms and at various levels within the epidermis/dermoepidermal junction. Examples of these mechanisms include the following: exocytosis/spongiosis, formation of subcorneal spongiform pustules, cytolysis and keratinocytic necrosis, antiepidermal antibody formation, deposition of immunoglobulin at the basement membrane zone, and photo-induced collagen alterations that lead to a mechanobullous disorder. Most bullous drug reactions are the result of an immunologically mediated inflammatory response, although pseudoporphyria cutanea tarda (pseudo-PCT) is not associated with significant inflammation. Studies have reported the preferential activation of drug-specific CD8+ T cells in the pathophysiology of some bullous drug eruptions.
Etiology
Contact sensitization to certain topical medications may result in a predisposition to a systemic eczematous reaction to the same or a chemically related medication. Also note the following:
-
Contact sensitivity to penicillin may cause a diffuse eczematous reaction to systemically administered penicillin or even the small amounts of penicillin in cow's milk taken orally.
-
Contact sensitivity to topical sulfonamides may cause a reaction to systemically administered sulfamethoxazole or sulfonylureas (ie, tolbutamide, carbutamide) but not to dapsone or sulfapyridine.
-
Contact sensitivity to ethylenediamine found as a preservative in some topical medications may predispose an individual to a reaction to systemically administered aminophylline, theophylline, tripelennamine, antazoline, methapyrilene, hydroxyzine, and pyrilamine.
-
Contact sensitivity to tetramethylthiuram disulfide predisposes a person to a reaction to the antialcohol treatment Antabuse (tetraethylthiuram disulfide).
-
Patients sensitized to paraphenylenediamine may react to azo dyes taken orally or the group of para drugs.
-
Other drugs that may cause an eczematous eruption but are not preceded by contact sensitivity are the following: carbamazepine, gold, griseofulvin, phenytoin, piroxicam, thiazide diuretics, and vitamin K.
-
One case of a bullous systemic contact dermatitis occurred after an intra-articular injection of a corticosteroid. [7]
-
An eczematous reaction has been reporedt to occur after intravenous immunoglobulin injections. [8]
-
Ustekinumab treatment has been reported to induce an eczematous drug eruption. [9]
The drugs most commonly implicated in causing AGEP are antibiotics, especially beta-lactams, macrolides, and cotrimoxazole. Ciprofloxacin has been reported in induce a bullous form of AGEP. [10] Furosemide and nonsteroidal anti-inflammatory agents have also been reported to be associated with the development of AGEP. [11] Diltiazem has been reported to cause AGEP several times. Other causes include acyclovir, [12] carbamazepine, hydroxychloroquine, [13] clindamycin, ticlopidine, terbinafine, high-dose chemotherapy, chromium picolinate, chloramphenicol, sulfapyridine, metronidazole, lacquer chicken, protease inhibitors, progesterones, mercury, nystatin, amoxapine, paracetamol, chloroquine and proguanil, nifuroxazide, lansoprazole, minocycline, dexamethasone injection, propicillin, aspirin, doxycycline, furosemide, and buphenine.
Many drugs are capable of causing FDEs. Some of the more common etiologic agents of FDEs include aspirin, barbiturates, cotrimoxazole, phenolphthalein, feprazone, sulfonamides, and tetracycline. [14] Causative agents in generalized bullous FDEs include aminophenazone, antipyrine, barbiturates, co-trimoxazole, diazepam, mefenamic acid, [15] paracetamol, phenazones, phenylbutazone, piroxicam, sulfadiazine, and sulfathiazole. An extensive bullous fixed drug eruption was reported after the second dose of the mRNA-1273 (Moderna) COVID-19 vaccine. [16] Knowledge of the potential drugs involved in a FDE is especially important because certain drugs have a predilection to cause FDEs at certain sites. Aspirin has a predilection for the trunk and limbs, tetracyclines for the genitalia, and phenylbutazone for the lips.
No reproducible tests for the etiology of EM exist. Association with infectious agents, such as herpes simplex and mycoplasma, has been well described. Precipitation of SJS or TEN has most commonly been associated with certain medications. The most commonly associated medications are the following: antibiotics (eg, sulfonamides, trimethoprim-sulfamethoxazole, penicillins, cephalosporins, chloramphenicol, clindamycin, griseofulvin, rifampin, streptomycin, tetracycline, clarithromycin, [17] ciprofloxacin [18] ), nonsteroidal anti-inflammatory agents (eg, ibuprofen, acetylsalicylic acid, ketotifen, naproxen, [19, 20] piroxicam, sulindac), antihypertensives, anticonvulsants (eg, phenobarbital, carbamazepine, phenytoin), and allopurinol. More recently, COX-2 inhibitors have been reported to be associated with SJS. [21] Sildenafil has been implicated in the development of EM minor in an HIV-positive patient and confirmed with patch testing. [22]
Topical mechlorethamine reportedly caused a subepidermal bullous reaction in a patient with mycosis fungoides. [23]
Methotrexate has been reported to be associated with bullous acral erythema in a child. [24]
The thiol group of drugs is the most common agent implicated in drug-induced pemphigus. Drugs known to cause pemphigus include amoxicillin, ampicillin, captopril, [25, 26] cephalosporins, penicillamine, penicillin, pyritinol, rifampin, and dipeptidyl peptidase inhibitors (DPP-4i) or gliptins for diabetes treatment. [27] Thiol drugs are more likely to cause pemphigus whereas nonthiol drugs are more likely to trigger pemphigus. For this reason, spontaneous recovery is lower in non–thiol-induced pemphigus where other factors may be predisposing a patient to develop pemphigus. Captopril has been reported to cause lichen planus pemphigoides. [28] There is a reported case of a bullous pemphigus reaction to the second dose of the mRNA COVID vaccine, in a 78-year-old male in Saudi Arabia. [29] Guselkumab, a biologic drug for plaque psoriasis treatment, was responsible for drug-induced pemphigus in 1 patient. [30]
A 2020 systematic literature review concluded that drug-induced pemphigus is most commonly seen with gliptins, PD-1/PD-L1 inhibitors, loop diuretics, penicillin, and penicillin derivatives. [31]
Sulfur-containing drugs commonly cause drug-induced pemphigoid, with furosemide being the most common cause. Other agents commonly known to cause drug-induced pemphigoid include amoxicillin, ampicillin, phenacetin, penicillin, penicillamine, psoralen-ultraviolet-A light, and beta-blockers. [32] One case of bullous pemphigoid was induced by an m-TOR (mammalian target of rapamycin) inhibitor in a renal transplant recipient. [33] Cicatricial pemphigoid has occurred after the use of drugs including practolol, topical echothiophate, D-penicillamine, clonidine, topical pilocarpine, topical demecarium, indomethacin, topical glaucoma, and sulfadoxine. Oral terbinafine has been associated with the development of bullous pemphigoid. [34]
Vancomycin is the most common cause of drug-induced LAD. [35, 36, 37] Other drugs known to cause LAD include diclofenac, somatostatin, lithium, phenytoin, captopril, amiodarone, cefamandole, amoxicillin, [38] and ampicillin-sulbactam. [39] Sulfasalazine was reported to cause one case of linear immunoglobulin A (IgA) bullous dermatosis. [40]
True PCT may be precipitated by barbiturates, estrogens, griseofulvin, rifampicin, and sulfonamides. The drugs that are known to induce pseudoporphyria include furosemide, nabumetone, nalidixic acid, naproxen, oxaprozin, tetracycline, and voriconazole. [41]
Epidemiology
Frequency
United States
Overall incidence of adverse cutaneous reactions to drugs has been estimated at 0.1-2.2% of treatment courses; however, semisynthetic penicillins and sulfamethoxazole/trimethoprim may have a considerably higher incidence at 3-5% of treatment courses. Patients infected with HIV may be at greater risk for adverse cutaneous drug reactions. Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have an incidence ranging from 1.8-9 cases per million and are more frequent in those younger than 20 years and older than 65 years.
International
The United Kingdom, France, Germany, and Italy have reported similar incidences of drug reactions and TEN as the United States. However, one survey in the United Kingdom found that only 2-10% of serious reactions are reported.
Race
Bullous drug reactions have no racial predilections.
Sex
In general, adverse drug reactions occur more commonly in women, although erythema multiforme (EM) has been reported to occur more frequently in men.
Age
Elderly patients who take multiple medications are at higher risk for the development of adverse drug reactions. Young men seem to be at higher risk for EM.
Prognosis
Most bullous drug eruptions resolve without significant sequelae once the offending drug is removed. However, the morbidity of these reactions is proportional to the extent of skin surface area and mucous membrane involvement.
Of patients who develop TEN, 25-30% die. Elderly patients have a higher mortality rate with TEN. Sepsis is the most common cause of death in TEN. SJS and TEN may result in a residual cutaneous pigmentary disorder and possible scarring of the ocular mucosa in those who survive.
Eczematous or spongiotic drug reactions usually have a good prognosis and resolve without significant sequelae.
AGEP has a good prognosis and resolves without sequelae once the causative agent is removed.
Bullous generalized FDEs have a favorable prognosis.
EM most often has a good prognosis, but SJS and TEN can be lethal depending on the extent of skin involvement and the age of the patient.
Pemphigus has a mortality rate approaching 10%. However, drug-induced pemphigus usually resolves with removal of the offending agent. In some patients, lesions may progress or persist. In these cases, the drug likely is serving as a trigger rather than a cause in patients who are already prone to develop pemphigus.
Drug-induced pemphigoid has an excellent prognosis with discontinuation of the drug. However, some cases may involve persistent lesions. Cicatricial pemphigoid, in comparison to idiopathic bullous pemphigoid, shows a small tendency for remission. In severe cases of ocular cicatricial pemphigoid, scarring and blindness in both eyes has been reported.
Drug-induced LAD has a good prognosis.
Drug-induced PCT has a good prognosis.
-
Small pustules on erythematous patch (acute generalized exanthematous pustulosis).
-
Annular hyperpigmented patch (fixed drug eruption).
-
Target or iris lesions on palm (erythema multiforme).
-
Coalescing eroded patches (Stevens-Johnson syndrome).
-
Stevens-Johnson syndrome.
-
Crusted erosions on scalp (drug-induced pemphigus).
-
Small vesicle at edge of urticarial plaque (drug-induced pemphigoid).
-
Tense vesicles in annular array (linear immunoglobulin A dermatosis).
-
Erosions, scars, milia, and vesicle (pseudoporphyria).