Products
Inferior vena cava (IVC) filter placement is most commonly indicated for deep venous thrombosis (DVT) or pulmonary embolism (PE) when anticoagulation therapy is contraindicated.
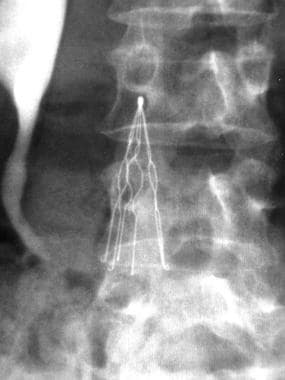
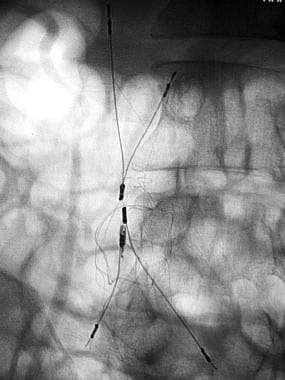
The first image below demonstrates an IVC of normal size without a thrombus, but there is a circumaortic left renal vein and an inflow defect at the origin of the right renal vein. If the infrarenal segment of the inferior vena cava is too short for a filter placement, the filter should be placed above the renal veins. The second image below shows one type of IVC filter (titanium Greenfield filter) in place.
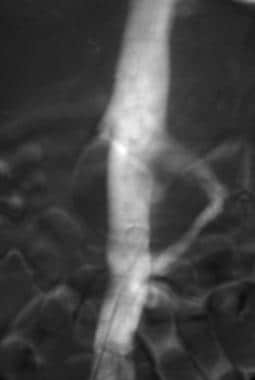 Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates an inferior vena cava of normal diameter without thrombus. In addition, the inflow from the renal veins can be seen at the level of the L1 vertebral body.
Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates an inferior vena cava of normal diameter without thrombus. In addition, the inflow from the renal veins can be seen at the level of the L1 vertebral body.
Category
Inferior vena cava filters
Device details
Permanent filters
Bard Peripheral Vascular
-
Simon Nitinol Filter (see the following image)
B Braun Medical
-
VenaTech LGM vena cava filter
-
VenaTech LP vena cava filter
Boston Scientific
-
Stainless steel Greenfield filter (SGF) (Kimray-Greenfield filter)
-
Titanium Greenfield filter (TGF)
-
Percutaneous stainless steel Greenfield filter (12F SGF)
-
An example of an inserted Greenfield filter is shown in the image below
Cook Medical
-
Gianturco-Roehm Bird's Nest filter (BNF) (see the following image)
Cordis
-
TrapEase permanent vena cava filter (see the image below)
Rafael Medical Technologies
-
SafeFlo vena cava filter
Retrievable/optional filters
ALN Implants Chirugicaux
-
ALN optional vena cava filter
Bard Peripheral Vascular
-
Recovery vena cava filter (discontinued)
-
G2 vena cava filter
-
G2 X vena cava filter
-
Eclipse vena cava filter
Cook Medical
-
Günther Tulip vena cava filter
-
Celect vena cava filter
Cordis
-
OptEase retrievable vena cava filter
Rex Medical
-
Option retrievable vena cava filter (previously licensed by Angiotech; currently licensed by Argon Medical Devices)
Design Features
General considerations
Desired quality of IVC filters
Inferior vena cava (IVC) filters are designed for their physical properties, clot-trapping effectiveness, ability to preserve flow in the IVC, and ease of placement. Of the currently available filters, each type has some but not all of the following properties or capabilities:
-
The filter should be able to trap most, if not all, thrombi to prevent new or recurrent pulmonary emboli (PE)
-
The filter should be nonthrombogenic and should maintain caval patency
-
The filter should be made of a biocompatible material that is durable and noncorrosive
-
The filter's shape and structural integrity should be maintainable for a long time
-
The filter's delivery system should have a low profile and allow easy placement
-
Clot trapping should be reasonably effective even if the filter deployment is suboptimal
-
The filter should not migrate after deployment
-
No perforation of the IVC should occur
-
The filter should be nonferromagnetic to allow magnetic resonance imaging (MRI) to be performed after its placement
-
The filter may be retrievable
Technical considerations of filter placement
Mobin-Uddin umbrella and 24F Kimray-Greenfield filters were originally designed for placement through a surgical venotomy. All of the other currently available filters can be placed percutaneously.
According to manufacturer recommendations, certain filters cannot or should not be placed by using certain approaches. When filter design allows placement through the jugular or femoral veins, the filter is specially packaged to ensure that it is deployed in the correct orientation.
Most filter placement procedures are performed in the angiographic suite with fluoroscopic guidance. In recent years, IVC filters have been placed successfully at the bedside in trauma patients in critical condition. [1] The procedures may be performed under guidance with a C-arm portable radiographic unit or with an intravascular ultrasonographic (US) scanner.
Most filters are placed in the infrarenal IVC. On the basis of the knowledge gained from caval ligation, some investigators advocate placement of the apex of any filter specifically within the inflow from the lowest renal vein to minimize the dead space between the filter and the renal veins. Suprarenal filter placement, when necessary, has been proven to be safe. Placement of a suprarenal IVC filter may be indicated in the following situations:
-
Renal vein thrombosis
-
IVC thrombosis extending above the level of the renal veins
-
Thrombus in the infrarenal IVC that does not leave enough room for the filter between the thrombus and renal veins
-
Recurrent PE despite infrarenal filter placement (in which upper extremity or superior vena cava [SVC] thrombosis should be excluded)
-
PE after ovarian vein thrombosis
-
Pregnancy
Regarding SVC filter placement, upper-extremity deep vein thrombosis (DVT) accounts for fewer than 5% of cases of venous thrombosis. The incidence has increased since the 1960s because of the more common use of indwelling catheters. Central venous catheters are responsible for 17-47% of reported upper-extremity DVTs. PE develops in as many as 12% of patients with upper-extremity venous thrombosis. Treatment options include rest and elevation of the affected arm, anticoagulation therapy, and thrombolysis. Filters have been deployed in the SVC and can effectively prevent symptomatic or fatal PE.
Placement of an SVC filter requires use of a modified technique. To maintain the correct orientation of the filter, a filter designed for jugular access is placed via a femoral approach, whereas the filter designed for femoral access is placed via a jugular approach.
It is more technically demanding to perform percutaneous insertion of an SVC filter than placement of an IVC filter, because the SVC is shorter and has a relatively smaller area for safe filter deployment. However, in reported series, no evidence of filter migration, dislodgement, or fracture was observed.
IVC filters
Mobin-Uddin umbrella filter
The Mobin-Uddin umbrella filter was the first true intravascular device and is included here for historical interest. This filter was discontinued in 1986.
Greenfield filter
In 1973, Greenfield et al introduced a new stainless steel filter. [2] The Greenfield filter has been on the market the longest, it is the most extensively evaluated filter, and it has become the criterion standard with which other IVC filters are compared. (See the image below.)
The original Greenfield filter is conical, and it consists of 6 strands of 0.015-in, zigzag-shaped, 316L-grade stainless steel legs in a radial array, each with a hook at the end that anchors the filter to the IVC. The steel struts are 4.6 cm in length and are fitted to an apical hub, which is directed cephalad in the patient. The base is 3.0 cm in diameter with the legs separated by 11 mm in the deployed position. The filter is loaded into a 24F carrier with a 29.5F external diameter. Initially, the filter was placed via a surgical cutdown of either the right internal jugular vein or the right common femoral vein. Tadavarthy et al were the first to describe percutaneous placement of the Greenfield filter in 1984. [3]
The design of the Greenfield filter made it possible for thrombi to fill and occlude 70% of the filter cone, representing a volume of thrombus of approximately 4 cm3 (34.3% of total volume) without reducing the cross-sectional area by 50%. If the cone is filled to 80% of total volume, the reduction of cross-sectional area is 64%. The ability of this filter to trap clinically significant emboli has been demonstrated in several in vitro and in vivo studies.
In 1995, Greenfield et al reported on their 20-year experience with the Greenfield filter. [4] Recurrent PE was seen in 4% of 642 filter placements. The rate was comparable to that reported by other authors. Athanasoulis et al reported an overall recurrent PE rate of 4.9% in their experience with 7 different designs. [5] In the group of patients receiving the standard 24F Greenfield filter, 38 (8.4%) of 455 patients had recurrent PE. [5] Kantor et al reported access site thrombosis in 7 (41%) of 17 patients; this rate of thrombosis was associated with the large size of the delivery system. [6]
In 101 patients evaluated, Greenfield et al reported patency of the insertion site in 94 patients (93%). [4] In the same series, the authors reported an IVC patency rate of 96%. Migration of the filter was reported in 30-49% of patients. Allowing for respiratory variation, Greenfield et al readjusted the rate of migration of more than 3 mm to 8%. [4] Filter tilt, crossing of limbs, and limb fracture occur in a low percentage of patients, and most of these events are not associated with clinical sequelae.
Extension of the filter struts beyond the IVC, which is suggestive of perforation, has been noted in high incidence on computed tomography (CT) scanning. Proctor et al performed animal studies to evaluate the apparent vena caval penetration by the Greenfield filter. [7] All filters appeared to penetrate the IVC on cavography and CT scanning. Laparoscopy failed to demonstrate penetration, and histology revealed remodeling of the intimal surface of the IVC and thinning of the adventitia. [7] The authors further hypothesized that the presence of the filter caused adaptation and remodeling of the IVC to occur.
Titanium Greenfield filter
The titanium Greenfield filter (TGF) was introduced in 1989 as a low-profile system to facilitate the ease of percutaneous insertion. It is made of a titanium alloy with elastic properties that allow the filter to be fitted into a 12F carrier system, as opposed to the 24F stainless steel Greenfield filter (SGF). The design of the filter preserves the conical shape consisting of 6 radiating, zigzag legs.
The TGF measures 47 mm; by contrast, the SGF measures 46 mm. The base of the TGF is 38 mm, as compared with 30 mm in the SGF. At 0.25 g, the TGF is lighter than the SGF, which weighs 0.56 g. The material is resistant to flexion fatigue and corrosion, and is nonthrombogenic and nonferromagnetic. The filter can be inserted via either the right or left femoral or jugular vein through a dilator system with an attached 14F sheath. The femoral and the jugular systems are supplied with differently coded packages.
During their preliminary clinical studies, Greenfield et al found an unacceptable 30% rate of migration, tilting, and penetration. [8] The TGF has greater lateral force at the base than the SGF, which may result in a higher rate of IVC penetration. The hooks have been modified through development and evolution. [9] Experience with the TGF with modified hooks (TGF-MH) was reported in 1991. Migration was greater than 9 mm in 11% of patients. [10] There was an increase in base diameter by greater than 5 mm in 14% of patients (with 1 filter penetration confirmed with CT scanning). Venous thrombosis at the insertion site occurred in 8.7% of patients. [10]
The TGF demonstrates a tendency for crossing 1 or more pairs of filter struts. Greenfield et al reported this occurrence in 8.7% of 181 cases in 1991 [11] ; the authors reported a 10% rate of filter tilt in 1994. [12] Sweeney et al reported filter tilting in 23% of patients. [13]
In vitro and clinical studies by Greenfield et al showed that clot-trapping ability and the rate of recurrent PE appeared not to be affected adversely by filter tilt or leg distribution. Other authors suggest that the jugular approach resulted in less filter tilt than the femoral approach. The TGF provides the same degree of clot-trapping ability as the stainless steel 24F Greenfield filter. The overall recurrent PE rate was reported to be 3.2-5%, which is comparable to the rates seen with other filter designs. [14] The IVC patency rate after TGF insertion was 97.8% in a long-term follow-up study of 373 patients by Greenfield et al; insertion-site thrombosis was seen in 2% of patients. [14]
Percutaneous stainless steel Greenfield filter
In the course of the continual improvement of the Greenfield filter design, a 12F stainless steel over-the-wire Greenfield filter was developed to provide a lower-profile introducing system. [11] The hook design was improved to prevent filter migration. The guidewire is used during deployment of the percutaneous steel Greenfield filter (PSGF) to provide a centering effect during filter release. Greenfield et al suspected that elimination of the guidewire may have been responsible for the high incidence of tilt of the TGF. The US Food and Drug Administration (FDA) approved the PSGF in 1995.
The PSGF, also called the stainless steel Greenfield filter with alternating hooks (SGF-AH), is manufactured from the same 316L-grade stainless steel as the original 24F SGF, and it retains the conical shape with 6 zigzag radiating legs. [15] The legs are welded to the apical bead, now at an angle of 0° as opposed to 17.5°. An opening in the center of the apical bead allows passage of a guidewire. The legs are 4.9 cm long (vs 4.7 cm for the TGF and 4.6 cm for the standard Greenfield filter). The base diameter is 3.2 cm (vs 3.8 cm for the TGF and 3.0 cm for the standard Greenfield filter). The system is preloaded differently for jugular and femoral approaches through a 12F carrier system.
Fluoroscopic control during withdrawal of the guidewire was recommended, because the guidewire can get caught between the legs near the apex or the hook. The not infrequent entrapment of guidewires was believed to result from the more crowded legs toward the apex with this new design. In 1998, Johnson et al reported that 21 (55%) of 38 patients had filter tilting of more than 15°, as evaluated with postplacement cavography and CT scanning. [16] The authors found a significant difference between filters placed via the jugular approach (12%) and filters placed via the femoral approach (51%). [16] Kinney et al also found that the right jugular approach resulted in the least filter tilt in their experience with 104 PSGF placements. [17]
In 2000, Greenfield et al reported the results of 600 PSGF placements in 599 patients. [18] Among patients receiving the PSGF with the modified hook, they found filter tilt in only 0.4%. Filter tilt was present in 5% of patients receiving the PSGF with standard hooks. Filter migration of more than 20 mm was seen in as many as 27% of PSGF placements with the standard hook but in less than 1% of the PSGF placements with alternating hooks. [18] Recurrent PE was reported to occur in 2% and 2.6% by Johnson et al and Greenfield et al, respectively. Greenfield et al reported a caval patency rate of 98% and a 4.3% rate of insertion thrombosis.
Bird's Nest filter
The Bird's Nest filter (BNF) was developed in 1982. This filter is constructed of a network of 4 biocompatible stainless steel wires (see the following image). Each wire is 25 cm long and 0.18 mm in diameter. The wires are preshaped with many nonmatching bends of a short radius and they are fixed at each end to V-shaped struts, the 2 legs of which are connected at an acute angle. A hook with a small loop stop minimizes the risk of IVC perforation at the end of each strut. The original designs called for 0.25-mm struts, but the later design (introduced in 1986) used 0.46-mm struts. This change increased the size of the loaded filter from an 8F to a 12F catheter system. The length of the introducer is 75 cm for the jugular approach and 40 cm for the femoral approach.
When a Bird's Nest filter is deployed, one of the V-shaped paired struts is pushed gently to engage to the IVC wall. Originally, it was recommended that the catheter be withdrawn by 1-3 cm over the pusher wire after the hooks were fixed to the IVC; later, it was recommended that 2 or 3 twists of 360° be applied. The purpose of the twists is to prevent or reduce the chance of wire prolapse. The second pair of struts is then pushed into the IVC so that the junctions overlap by 1-2 cm. The handle of the pusher wire is turned counterclockwise 10-15 times to free it from the struts.
The Bird's Nest filter has a unique design. The final shape of the deployed filter varies owing to the flexibility of the long 0.18-mm wires. The maximum span of the V-shaped struts is 60 mm. Therefore, the Bird's Nest filter is suitable for placement in a megacava with a diameter greater than 28 mm, [19] and the FDA approved it for this use. Other filters are associated with a risk of migration if the IVC diameter is greater than 28 mm. The Bird's Nest filter has been placed successfully in patients with an IVC diameter as large as 42 mm, without filter migration.
In an experimental model, Korbin et al suggested that placement of a single Bird's Nest filter was preferred to bi-iliac placement of filters in cases of megacava. [20] Other investigators also demonstrated the clot-trapping efficacy of the BNF in various in vitro studies. [19, 21, 22, 23, 24]
Wire prolapse beyond the struts has been reported, but it does not affect the clot-trapping ability of the Bird's Nest filter. CT scan studies performed in patients with Bird's Nest filters demonstrated penetration of the caval wall in 83%. All of the patients were asymptomatic in the series reported by Wojtowycz et al. [25] The authors also reported that 8 patients (3%) in the series of 267 Bird's Nest filter insertions had thromboses at the access site.
Roehm et al reported a rate of 2.9% for IVC thrombosis in their series of 568 patients. [26] IVC thrombosis was reported to occur in as many as 17% of patients by Ferris et al, [27] in 7% by Thomas et al, [28] and in 4.7% by Nicholson et al. [29] Wojtowycz et al reported rates of 1% confirmed and 3% suspected recurrent PE, which is similar to the 2.7% rate reported by Roehm et al. Athanasoulis et al reported a 7% recurrent PE rate, 90% of which were fatal, in a series of 255 patients. [5]
Vena Tech LGM filter
The Vena Tech LGM filter was first developed and released in France by LG Medical; FDA approval was obtained in 1989. The filter is stamped and point-welded and manufactured from a biocompatible, nonferromagnetic metal alloy called Phynox (cobalt, chromium, iron, nickel, molybdenum). The filter is conical with 6 radiating, flat but slightly curved legs joining a central hub at the apex. At the base of the filter, each leg is attached to a flat side rail that contacts the wall of the IVC and thus acts as a vertical stabilizing strut.
The shape of each leg–side rail assembly resembles a skewed V or a check mark. A small spike is stamped approximately 13 mm from the cephalad end of each side rail, which provides longitudinal stability and prevents filter migration. In the original design, the side rails were 34 mm in length and the legs were 45 mm in length. In the latest design (introduced in 1991), the legs are 35.5 mm in length. The open filter is 38 mm in height. The base diameter is 30 mm and is suitable for deployment in IVCs as large as 28 mm in diameter. [30, 31] However, Millward et al reported successful insertion of a Vena Tech-LGM filter without significant migration in 4 patients with IVCs greater than 28 mm in diameter. [32] The filter is delivered through a 12F introducer sheath from either a jugular or femoral approach.
Incomplete opening of the Vena Tech LGM filter has been shown to affect the clot-trapping ability of the filter. In 1991, Reed et al reported an incomplete opening of the filter of as much as 41% when it was placed by the jugular approach, and they recommended that the filter not be inserted via this approach. [33] Murphy et al reported incomplete opening of the filter in 6% of filter insertions. [34] Rapid deployment by brisk retraction of the insertion cannula has been recommended to avoid incomplete opening.
A rate of as much as 6% of recurrent PE was reported in a number of long-term follow-up studies after insertion of the Vena Tech LGM filter. Of these patients, fatal PE occurred in 25-50%. The incidence of thrombosis at the insertion site was reported to be 8-23%. Murphy et al demonstrated a rate of 37% for filter thrombosis, with cephalad extension of thrombus seen in 20%. [34] Crochet et al reported a progressive decrease in IVC patency, with a rate of 66.8% at 9 years into the follow-up period. [35] Filter placement resulting from failed anticoagulation therapy for PE appeared to be the main predictor of IVC occlusion. Earlier, the Crochet et al reported IVC patency rates of 92% after 2 years, 80% after 4 years, and 70% after 6 years of follow-up monitoring. [36] In the same study, the authors reported an 18% rate of filter migration.
Vena Tech LP filter
The new Vena Tech filter design was approved by the FDA in 2001. Similar to the LGM, the Vena Tech LP filter is made from Phynox and, therefore, is MRI-compatible. This filter has a conical design with 8 wires for clot trapping connected to 4 sets of inverted V-shaped struts with anchoring barbs. A single design is packaged for both femoral and jugular approaches, with color-coded pushers and matching locking mechanisms for the different approaches. A newer 96-cm introducer kit for an antecubital approach is available. The introducer system is by a 9F outer diameter (OD) (7F inner diameter [ID]) sheath. When deployed, the height is 43 mm. The Vena Tech LP filter was approved by the FDA for use in patients with IVCs of diameters up to 28 mm; in Europe, it is used in patients with IVCs of up to 35 mm in diameter.
In a 2008 report on a multicenter experience in 106 patients, Le Blanche et al found preexisiting partial caval thrombosis in 5 patients and IVC occlusion in 2 patients during the first follow-up period, but no new events during the subsequent follow-up. [37] Filter tilt greater than 15° was seen in 2 patients at the 30-day follow-up. During the 3 follow-up periods, no recurrent or new PE, filter fracture, or migration was seen. [37]
Simon nitinol filter
Nitinol is an alloy consisting of 53% nickel, 45% titanium, and 2% cobalt that was developed in the 1960s at the US Naval Ordnance Laboratory. It is pliable at room temperature but has thermal memory and reverts to its preformed shape at body temperature. Nitinol resists corrosion, is nonferromagnetic, and is stronger than steel.
Simon et al performed experimental studies with this material in the 1970s, and ultimately, they developed a filter that was approved by the FDA in 1990. [38] The filter is 3.8 cm long and has 2 sections. The filter mesh, which is directed toward the heart, consists of 7 overlapping loops of 0.015-in–diameter (0.038-cm–diameter) nitinol wires fused together at 2 points. This part has an outer diameter of 28 mm. The anchoring basal part of the filter is formed by 6 diverging limbs in a radial array. Each has a small hook at the end that engages the caval wall for a depth of 1 mm (see the image below).
The deployed filter can adapt to an IVC with a diameter as large as 28 mm. The legs also function as a coarse filter; the dome forms a fine filter and is designed to trap emboli larger than 5 mm, as well as a significant portion of smaller emboli. In vitro studies have demonstrated that the Simon nitinol filter traps more of the smaller clots than the other filters. Compared with the Greenfield filter, clot-trapping capability is not affected by filter tilt.
The Simon nitinol filter can be placed via the femoral, jugular, and antecubital [39] approaches. The delivery system for the jugular kit is longer, and the orientation of the filter is reversed in comparison with that in the femoral kit. Initially, a cold saline infusion through the storage tube via the side port was recommended to soften the filter. Later, modification of this filter eliminated the need for cold saline infusion. As a result of the flexibility of this filter at low temperature, placement through the right external jugular vein and right antecubital vein approaches have been successful using the internal jugular kit. Upon deployment, shortening of approximately 1-2 cm occurs as the dome of the filter is formed. In patients with small IVCs, incomplete formation of the dome can be seen after deployment; this does not alter filter function.
In a long-term study of 114 consecutive Simon nitinol filter insertions, Poletti et al reported a rate of PE recurrence of 4.4%. [40] Comparing different filters used during the past 26 years, Athanasoulis et al reported that the Simon nitinol filter had the lowest rate for recurrent PE (3%) and fatal recurrent PE (2%). [5] Poletti et al also reported a 3.5% rate of IVC thrombosis and a 3.5% rate of thrombosis at the femoral vein access site. [40] Aswad et al reported a higher incidence of access site and IVC thrombosis, but the rate was not significantly different from rates compared with the other types of filters studied. [41]
In a series of 103 patients, Simon et al found 5 patients with nonoccluding thrombus in the IVC, as well as 3 patients each with asymptomatic and symptomatic IVC occlusion. [42] Using MRI, Grassi et al raised the possibility of malignancy contributing to IVC thrombosis in 50% of patients with underlying malignancy undergoing Simon nitinol filter placement. [43] Filter migration or IVC perforation was not seen by Simon et al in their series of 103 patients. [42] In addition, Poletti et al found no filter migration in 38 patients in a study with a mean follow-up period of 32.2 months. [40] Strut fracture, axial deviation of the filter, and IVC perforation have been reported with the Simon nitinol filter; however, none of these complications appears to affect filter function adversely.
TrapEase filter
The TrapEase filter was approved by the FDA in the summer of 2000. The filter is laser cut from a single tube of nitinol material without welding. It has a symmetric, trapezoidal, double-basket design providing 2 levels of clot trapping, as well as self-centering characteristics. The 2 baskets are connected through 6 side struts, each with proximal and distal hooks to provide filter anchoring. The TrapEase filter is 65 mm in length before deployment and 50 mm in length when expanded maximally to a diameter of 35 mm. The design allows placement in IVCs with diameters ranging from 18 to 30 mm. [22] (See the following image.) If appropriate, the catheter can be removed endoscopically. [44]
The unique symmetric design of the TrapEase filter allows placement from either the jugular or femoral approach by using the same 55-cm package. A separate 90-cm length package is suitable for the antecubital approach. The filter is deployed by a pusher through an 8F OD (6F ID) introducer set. Before the 90-cm kit was available, Davidson and Grassi reported 5 cases of successful antecubital placement, [45] and Stone et al reported successful placement on 39 patients via the subclavian veins. [46]
In 2001, Rousseau et al reported a 95% technical success rate from their clinical experience with the TrapEase filter in 65 patients at 12 centers in Europe and Canada; 3 filters were placed not at the intended site. [47] No filter migration, insertion site thrombosis, filter fracture, or IVC perforation was observed. At 6-months follow-up, no recurrent PE was identified. Two cases of filter thrombosis were reported. [47] Other authors reported similar results. Of 751 TrapEase filters placed, Kalva et al reported 2 cases (0.2%) of near-total caval occlusion and 15 cases (6.8%) of CT scan–confirmed PE in 219 patients. [48]
SafeFlo filter
The SafeFlo filter has a unique design; it is made of nitinol wires, and the anchoring mechanism is a double-ring platform. These 2 rings are oversized to exert radial force on the IVC and are slightly angled toward each other to provide additional bidirectional stability. This is connected to the filter element that is designed to be 3 mm smaller than the IVC. It is shaped from nitinol wires into an outer supporting ring and a 5-leafed inner filtration configuration.
The SafeFlo filter is offered in 3 sizes to fit IVC diameters from 15 to 27 mm. Deployment is through a 7F sheath via either a jugular or femoral approach.
Retrieval can be performed through the use of the VascuGrab retrieval system; however, the VascuGrab system is not yet approved by the FDA and not available in the United States.
In vitro results were previously published by Bruckheimer et al. [49] A mutlicenter clinical study has been performed, and the clinical results are pending.
Retrievable/optional IVC filters
Over the past decade, several new filters have been approved as retrievable filters. These devices are sometimes referred to as optional filters, because they can be retrieved after a period following placement or can be left in the patient as a permanent device. [50, 51, 52, 53, 54, 55, 56, 57, 58, 59]
Retrievable/optional IVC filters include the following:
-
Günther Tulip filter
-
Recovery filter
-
Recovery G2/G2 and G2 X filters
-
OptEase filter
-
Celect filter
-
ALN filter
-
Option filter
The SafeFlo filter is available in Europe as an optional filter, but it is currently approved only as a permanent filter in the United States.
There are also temporary filters that require removal, such as the Tempo filter II. Convertible filters are optional filters that the filtration property can be altered with a second procedure. Such an example is the VenaTech Convertible filter, which becomes a stent after conversion. These are currently not available in the United States.
Some patients need a filter to protect from PE for only a short period. Decousus et al suggested that IVC filters provide only short-term protection. [60] Significant reduction of the PE rate was reported at day 12, but no difference was reported at 2 years when compared with anticoagulation therapy. After over 8 years of follow-up, the incidence of postthrombotic syndrome and of DVT were higher in the filter group, and the PE rate was lower. [60] In the same follow-up study by the Prevention du Risque d’Embolie Pulmonaire par Interruption Cave, a cumulative incidence of symptomatic IVC thrombosis of 13% after 8 years was noted [60] ; by contrast, Crochet et al reported a higher rate of 33% caval thrombosis in a 9-year follow-up of the Vena Tech LGM filter. [35]
The Society of Interventional Radiology guidelines for the use of retrievable filters recommends that "the decision to use an optional filter rather than a permanent filter should be based on the anticipated required duration of protection against clinically significant PE and/or risk of pharmacologic therapy." [54]
When the indication for caval filtration is no longer present in a patient or the risk of PE is acceptably low because of a change in clinical status or because prophylactic therapy was successful, these patients can be considered for filter retrieval. Patients should be fully evaluated by clinical status, laboratory findings, and imaging before the attempted retrieval.
When there is a need for continuous caval filtration, optional filters can be left in situ as a permanent device. In some cases, these filters are not removed or their retrieval may be deferred for various reasons, such as poor clinical status, short lifespan, significant amount of clot trapped within the filter, IVC thrombosis, or technical difficulties that prevent safe retrieval.
In younger patients who have a longer life expectancy, use of a retrievable filter is a more valid option for physicians and patients than the use of a permanent device. These optional filters have gained popularity over the years. More and more of these devices are being left as permanent filters. [61] Although the percentage of successful retrieval varies, some series quote retrieval rates of up to 100% technical success for the selected patients.
Günther Tulip filter
The Günther Tulip filter was approved by the FDA in 2003. [62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73] This filter is made from nonferromagnetic Conichrome, and it is conical in design with a hook at the apex for retrieval. It has 4 legs of 44 mm with barbed hooks for anchoring; 4 additional wires wrap around the primary legs to form a tulip-petal conical structure with a total of 12 wires for clot trapping. The maximum diameter is 30 mm, and its maximum height is 50 mm. The introducer system is of 8.5F ID, and different packages are available for femoral or jugular approaches. The retrieval system is of 7F ID (11F OD for the sheath) and is employed via a jugular approach only.
The direction of the hook is a factor that influences the rate of success during retrieval of the Günther Tulip filter. Millward et al recommend orientating the hook toward the lumen of the IVC, especially if filter tilting is anticipated. These authors also advocate anticoagulation for 24-48 hours before retrieval.
A venacavogram must be performed before retrieval to assess the amount of trapped clot. More than 25% of clot trapped in the cone is a contraindication to retrieval. The filter is also not to be retrieved in cases of continued requirement of a filter or if it is technically not possible to be retrieved because of trapped thrombus, adherence to the IVC wall, or filter tilt. The reported total caval occlusion rates are 0-6%.
The recommended retrieval time is in the first 12 days, but various authors have prolonged the time to retrieval with or without repositioning the filter for up to 3-4 weeks, or even longer. [74]
In 2009, Smouse et al reported on a multicenter experience of 554 patients. Filter retrieval was successful in 248 (90.2%) of 275 patients. [74] The mean indwell time was 58.9 days (range, 3-494 d). Their analysis showed a probability of successful retrieval greater than 94% at 12 weeks and greater than 67% at 26 weeks. [74] Excessive filter tilt and tissue in-growth are main causes of failed retrieval.
Recovery nitinol filter
The Recovery filter was approved by the FDA in 2003, but it is no longer available commercially and has been replaced by the G2/G2 X filters (discussed further below).
The Recovery filter is made from nitinol and is MRI-compatible. Similar to the Simon nitinol filter, it has 2 levels of filtration using 6 radiating arms and legs, each of 0.13-in nitinol wires. The legs have hooks for anchoring. The maximum diameter of the extended filter legs is 32 mm; it is approved for use in IVCs of up to 28 mm in diameter. The deployed height is 40 mm. The delivery system is of 7F ID (9F OD) through the femoral approach.
Retrieval is via a specific retrieval cone supplied as a separate kit. The system is introduced via the jugular route with a 12F sheath. The 20-mm cone has 9 steel struts within a urethane membrane with a base diameter of 15 mm when opened.
Asch reported successful retrieval of the Recovery filter without complications in 24 patients with a mean implantation period of 53 days (range, 5-134 d). [75] One migration of 4 cm occurred with a large, trapped thrombus. Grande et al reported recurrent PE in 2.8% of 106 Recovery filters placed [76] ; 14 filters were retrieved successfully from this group.
Kalva et al report their experience in 96 patients, in which retrieval was successful in 9 of 11 attempted patients. [77] The average indwell time was 117 days (range, 24-426d). On abdominal CT scan in 40 of the 96 patients, 12 (30%) showed clumping of the filter legs, and 11 (27.5%) showed penetration of the IVC walls.
In a series of 14 patients, Hull and Robertson reported arm perforation in 5 of 9 (56%) and leg perforation in 1 of 9 (11%) on early CT imaging (mean, 30 d; range, 0-126 d) and in all patients during final imaging (mean, 899 d; range, 119-1218 d). [78] They reported limb fracture and migration in 3 of their 14 patients (21%) and successfully performed late-stage retrieval on all the filters. [78] Cantwell et al reported 6 filter fractures in 128 patients. [79]
Recovery G2/G2 & G2 X filters
The Recovery G2 (or simply G2) and G2 X filters are modifications of the original Recovery filter. [80] These were approved by the FDA in 2008 and 2009, respectively.
The redesigned G2 filter has enhanced fracture resistance, better centering, improved fixation hooks, and longer legs with a wider span to reduce migration for IVCs up to 28 mm diameter. It is packaged separately for femoral and jugular approaches. The femoral kit has a 7F ID introducer sheath, whereas the jugular kit has a 10F ID set. The deployed height is 42 mm.
The retrieval set is the same as for the original Recovery filter. The G2 X filter has a hook at the apex, and is therefore retrievable by both the snare technique or with the cone retrieval kit.
Kim et al reported 5 new, symptomatic PE (6.5%) in 77 G2 filters placed. [50] In 2008, of 59 patients evaluated, Oliva et al reported 100% success for retrieval in 51 patients, with a mean dwell time of 53.4 days (range, 7-242 d). [81] Of these, 8 of 59 cases showed large trapped thrombi during cavography. Oliva et al reported 9 of 51 (18%) patients with IVC wall penetration and 6 of 51 (12%) patients with filter tilt greater than 15o. [81] There were no fractures or cranial migrations, but 2 caudal migrations occurred.
Charles et al reported filter tilt in 5 (18.5%) of their 27 patients. [82] In 2009, Binkert at al reported 15% filter tilts, 12% caudal migrations, and 1.2% filter fractures in 85 patients on a 6-month follow up. [83] Of the 61 patients referred for retrieval, 16 (26%) showed presumed penetration cavography, and 14 of 16 had successful retrieval. [83]
In the same year, Cantwell et al reported no filter fractures in their series of patients that compared the Recovery and G2 filters. [79] They further concluded that the G2 had less filter tilt but a higher rate of caudal migration than the Recovery filter. [79] Lynch and Kekulawela reported 6 (3.4%) filter fractures in 174 G2 filters placed, all of which were implanted for over 180 days. [84] They observed 76 of 174 (44%) cases of presumed caval penetration during removal. [84]
There have also been 2 reports of intraventricular migration. [85, 86]
OptEase filter
The OptEase filter is similar in design to the permanent TrapEase filter. It is cut from a single tube of nitinol, with a 6-sided, conical shape and dual-level clot-trapping capability. Although the OptEase filter can be deployed via either a femoral or jugular approach, the provision of a hook on the caudal end and unidirectional anchoring barbs to prevent cephalad migration allow retrievability from the femoral approach, which is unique to this design.
In 2002, this filter was approved by the FDA for retrieval times of up to 23 days. The introducer system has a profile similar to the TrapEase filter; the retrieval system is 10F.
Kim et al reported 11 cases (4.2%) of new PE and 1 case (0.4%) of IVC thrombosis in 260 OptEase filters placed. [87] Keller et al reported on removal of 58 of 80 OptEase filters placed, [88] in which 5 patients required additional access during retrieval, because the hooklet could not be engaged. The IVC thrombosis rate in their series was 3%. [88] No IVC thrombosis was seen in 27 patients reported by Oliva et al, [89] or in 94 patients reported by Rosenthal et al. [90] However, Corriere et al reported 3 (12.5%) cases of IVC thrombosis in 24 patients with retrievable filters; all 3 were OptEase filters. [91]
Celect filter
The Celect filter is a newer retrievable filter approved by the FDA in 2008. It is based on the Günther Tulip filter. The new design is made of the same material, Conichrome. The Celect filter has 4 anchoring struts for fixation and 8 independent secondary struts to improve self-centering and clot trapping.
The deployment kit is similar to that for the Günther Tulip filter, and it is available for both the femoral and jugular approaches. The same Günther Tulip filter retrieval set is employed for retrieval from the jugular approach.
Initial studies suggest that the retrieval interval of the Celect filter can be extended, with higher rates of successful retrieval. [92, 93]
In 2009, in a group of patients with Günther Tulip and Celect filters placed by intravascular ultrasonography (IVUS), Rosenthal et al reported filter tilt in 15 of 50 (30%) for the Günther Tulip filter and 6 of 65 (9.2%) for the Celect filter at cavography before retrieval. [94] Sangwaiya et al reported successful retrieval in 14 of 15 patients (93.3%) in a group of 73 placements, with a mean indwell time of 84 days (range, 5-381 d). [95] Two (2.8%) patients developed new PE after filter placement, similar in incidence to previous reports, and penetration of filter legs or arms was seen in 4 (22.2%). Fracture and migration of filter component, filter thrombosis, and iliac vein thrombosis were seen in 1 patient each (5.5%). [95]
In the same year, in a multicenter experience in 95 patients with Celect filter placement, Lyon et al reported 56 (96.6%) of the filters were successfully retrieved out of 58 patients with a mean indwell time of 179 days (range, 5-466 d). [96] They observed filter tilt greater than 15° in 5 (8.6%) patients at deployment and 2 (3.4%) patients at retrieval. Strut penetration was observed in 21 (36%) patients but no IVC perforation; 20 of these were retrieved successfully. [96] No significant filter migration, filter fracture, or IVC occlusion was observed.
ALN filter
The ALN filter has been available in Europe since 1997. It was approved by the FDA in 2008.
This filter is a conical filter made from 9 variable-length wires of 316L stainless steel. The upper level has 6 arms with hooks for anchoring and 3 longer legs for centering. The introducer sheaths are 7F, and the filters are preloaded in a holder. The filter kits are packaged separately for deployment for femoral, jugular, and brachial approaches.
Retrieval is via the jugular approach. The introducer set is 9F OD. The retrieval kit is a forceps design, cone-retrieval system with 8 stainless wires without any plastic cone.
In 2007, in a series of 220 patients with ALN filter placement with an 18-month follow-up, Mismetti et al reported filter tilt was seen in 12 of 212 (5.7%) during placement. [97] Fatal PE occurred in 5 (2.3%) within 24 hours to 11 days following placement. Filter retrieval was successful in 55 patients, with a mean indwell time of 51 days (range, 6-352 d); 4 of these patients required a second attempt because of filter thrombosis. [97]
In 2008, Pellerin et al reported successful retrieval was achieved in 122 of 123 patients (99%), with a mean indwell time of 93 days (range, 6-722 d). [57] Severe tilting was seen in 6 cases (5%), including 1 failure in retrieval. There was no filter migration, fracture, or IVC penetration. [57]
Option filter
The Option filter was approved by the in FDA in June 2009.
This is a laser-cut nitinol device designed for IVC diameters up to 32 mm. A somewhat unique feature is over-the-wire placement. The Option filter is a conical filter featuring 6 legs with hooks for anchoring, with a retrieval hook at the apex. The introducer set is 6F OD (5F ID), and the filter cartridge is color-coded for a femoral/jugular approach.
Clinical results are pending.
Indications
Deep venous thrombosis (DVT) and pulmonary embolism (PE) represent two points on the continuum of a single disease process. The reported annual incidence of DVT in the United States includes a wide range (250,000-20,000,000 cases of lower-extremity DTV involving treatment or hospitalization), and the disease constitutes a major healthcare burden.
Most commonly, DVT develops within the deep veins of the lower extremities, but it can also involve, or arise solely from, the veins of the pelvis or the upper extremities. The most feared complication of DVT is PE. PE frequently results in hospitalization, and it is associated with high morbidity and mortality rates. Currently, PE is the third leading acute cardiovascular cause of death in the United States. Death is associated with serious underlying disease in approximately 50% of patients with PE.
Systemic anticoagulation with intravenous heparin followed by oral warfarin remains the mainstay of treatment for DVT and for prevention of PE. However, studies have shown that as many as 33% of patients develop a second PE while receiving adequate anticoagulation therapy.
In addition, anticoagulation therapy is associated with hemorrhage, which precludes its use in certain groups of high-risk patients, including patients at high risk for falling, hemorrhagic stroke, central nervous system (CNS) metastasis, or bleeding diathesis. Although heparin can be used during pregnancy, warfarin crosses the placenta and has been shown to have significant adverse effects on the developing fetus.
The concept of interrupting the flow in the inferior vena cava (IVC) to prevent PE originated in the 1930s-1940s with the performance of ligation of the common femoral vein and superficial femoral vein. These methods were used in parallel with anticoagulation methods when heparin and warfarin became available in 1935 and 1948, respectively. Phlebotomy with clot removal was also performed if thrombus was present at the common femoral vein level. A high incidence of limb edema was associated with femoral vein ligation.
Later, ligation of the IVC was performed, initially in patients harboring thrombi above the superficial femoral veins. Eventually, it replaced lower-level venous ligation. The operative mortality rate of 2-15% was not significantly different from that for femoral vein ligation, but a lower rate for recurrent PE was reported. The optimal level of ligation was located immediately below the renal veins to prevent thrombosis caused by venous stasis between the interrupted IVC and the renal veins. However, 10-16% of these patients had immediate lower-extremity swelling.
In the 1960s, various methods of partially interrupting the flow in the IVC were developed to lessen the effect of venous stasis. The procedures provided emboli trapping with preservation of blood flow through the lumen. The procedures included suture plication and the use of caval clips (Moretz clip, Miles clip, Adams-DeWeese clip). The rates for operative mortality and recurrent PE were similar to those for caval ligation, yet the rate for limb edema was reduced. Despite the fact that these procedures partially interrupted flow in the IVC, caval occlusion rates of 30-40% were reported.
In 1967, the Mobin-Uddin umbrella filter (see Design Features) was developed as a replacement for surgical ligation, caval plication, and caval clips, as well as partially to interrupt the flow in the IVC and to prevent PE. Since then, several filters have been introduced. Currently, filters represent the standard of care when partial interruption of IVC flow is indicated to prevent the occurrence of PE.
DVT or PE in a patient with contraindication to anticoagulation therapy
Although the indications for IVC filter placement have expanded over the years, DVT or PE in a patient for whom anticoagulation therapy is contraindicated remains the most frequent indication, accounting for 38-77% of patients undergoing IVC filter placement.
Contraindications to anticoagulation therapy include the following:
-
Hemorrhagic stroke
-
Recent neurosurgical procedures or other major surgery
-
Major or multiple trauma
-
Active internal bleeding (eg, upper or lower gastrointestinal bleeding, hematuria, hemobilia)
-
Intracranial neoplasm
-
Bleeding diathesis (eg, secondary thrombocytopenia, idiopathic thrombocytopenic purpura, hemophilia)
-
Pregnancy
-
Unsteady gait or tendency to fall (as seen in patients with previous stroke, Parkinson disease)
-
Poor patient compliance with medications
DVT or PE in a patient with a complication of anticoagulation therapy
DVT or PE in a patient who has a complication of anticoagulation therapy accounts for 6-18% of IVC filter placements.
Anticoagulation therapy is not without potential complications; it is associated with a mortality of 5-12%, and therapy may have to be discontinued in as many as 18% of patients because of hemorrhage. Anticoagulation with heparin is also associated with immune-mediated heparin-induced thrombocytopenia (HIT) in 1-3% of treated patients. Approximately 25% of patients with HIT develop new venous and/or arterial thrombotic events, including PE.
Failure of anticoagulation therapy
Patients who have new-onset PE or DVT while they are receiving anticoagulation therapy are candidates for IVC filter placement. Among patients with PE, 3-33% have a second embolic event during adequate intravenous heparin therapy or oral warfarin anticoagulation treatment.
Free-floating iliofemoral or caval thrombus
Free-floating iliofemoral or IVC thrombus has been associated with a 27-60% rate of PE. Therefore, it is considered an indication for IVC filter placement. However, results have not been consistent. For example, Pacouret et al did not find a higher risk for PE in patients with free-floating proximal DVT. [98]
PE prophylaxis
IVC filter placement has been advocated as a means of preventing PE in patients at high risk for thromboembolic events. Traditionally, such patients have included the following populations:
-
Patients with DVT who are about to undergo surgery (lower-extremity orthopedic surgery, major abdominal surgery, neurosurgery)
-
Patients with chronic pulmonary hypertension and a marginal cardiopulmonary reserve
-
Patients with cancer
-
Trauma patients, including those with (1) severe head injury with prolonged ventilator dependence, (2) major abdominal or pelvic penetrating venous injury, (3) spinal cord injury with or without paralysis, (4) severe head injury with multiple lower-extremity fractures, or (5) pelvic fracture with or without lower-extremity fractures
In 1981, Moore et al reported 21 hemorrhagic events in 32 patients with malignancy who underwent anticoagulation therapy to treat thromboembolic disease. [99] Of these patients, 8 had hemorrhage resulting in cessation of therapy or death. This observation led to the conclusion that IVC filter placement is a safer means for PE prophylaxis. [99] However, this indication was disputed in 1998 by the American College of Chest Physicians Consensus Committee on Pulmonary Embolism [100] ; its report stated that the routine use of IVC filters is not recommended in patients with cancer and DVT or PE. [101]
Patients with severe trauma are prone to develop DVT and PE. In many of these patients, anticoagulation therapy is contraindicated because of the risk of hemorrhage. Other conservative methods, including compression devices and foot pumps, may not adequately prevent DVT. Although some studies have shown that the prophylactic placement of IVC filters prevents fatal PE in patients with trauma, other studies have not shown any significant reduction in the rate of PE. Additional studies of cost-effectiveness or risk-benefit considerations do not support prophylactic filter placement in patients with trauma. [102]
Contraindications
IVC filter placement has been shown to be safe, with only a few relative contraindications in some patients, such as the following:
-
Those receiving therapeutic anticoagulants
-
Those with thrombus between the venous access site and expected deployment site
-
Patients expected to undergo magnetic resonance imaging (MRI) after filter placement
Many of these contraindications have been addressed with changes in technique and the type of filter used. With smaller introduction and deployment systems and with the availability of ultrasonographic (US) guidance during venous puncture, the reversal of anticoagulation often is not necessary.
The presence of thrombus between the puncture and deployment sites requires the selection of a filter that allows an alternate approach (ie, brachial or jugular vein). The titanium Greenfield, Vena Tech-LGM, Vena Tech LP, Simon nitinol, TrapEase, OptEase, Günther Tulip, and Recovery filters are nonferromagnetic; therefore, for patients with these filters, subsequent exposure to the magnetic field during MRI is possible (see Design Features).
Clinical Implementation
This section discusses normal and variations of inferior vena cava (IVC) anatomy, preprocedural evaluations in assessing deep vein thrombosis (DVT) and pulmonary embolism (PE), and assessing the IVC for clinical implementation of IVC filters. See also Inferior Vena Cava Vascular Filter Placement.
Normal anatomy, IVC
The IVC is the largest venous structure in the body. It drains the venous return from the lower extremities, pelvis, and abdomen into the right atrium. The IVC originates at the L4-5 vertebral level and is formed by the confluence of the left and right common iliac veins. At this level, the IVC is posterior to the origin of the right common iliac artery. As it ascends to the right of the aorta and anterior to the spine retroperitoneally, the IVC receives blood from the lower lumbar veins and ascending lumbar vein.
The right gonadal vein and suprarenal veins empty directly into the IVC below and above the right renal vein, respectively. The counterparts of these veins on the left side drain into the IVC via the left renal vein. The renal veins join the IVC at vertebral level L1-2. The right renal vein has a shorter course and is typically situated more caudally. The left renal vein is longer and typically crosses in front of the aorta. At the level of the renal veins, the IVC lies posterior to the head of the pancreas. Then, it ascends in a groove posterior to the liver. The IVC receives the left, middle, and right hepatic veins, and often a separate branch from the caudate lobe, before it passes behind the right crus of the diaphragm to enter the right atrium (see the image below).
 Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates an inferior vena cava of normal diameter without thrombus. In addition, the inflow from the renal veins can be seen at the level of the L1 vertebral body.
Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates an inferior vena cava of normal diameter without thrombus. In addition, the inflow from the renal veins can be seen at the level of the L1 vertebral body.
Anatomic variations, IVC
Anatomic variations involving the IVC are fairly common in the general population, reflecting variation in the involution and persistence of the cardinal veins. These conditions may be important, because they alter the location of filter deployment in 3-15% of patients. A few of the more common conditions, especially those pertinent to IVC filter placement, are described below.
Duplication of the IVC
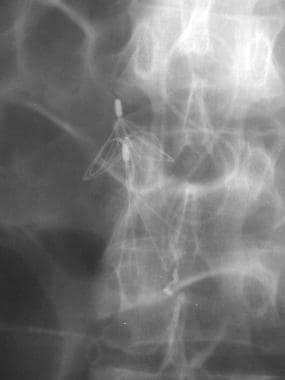
Duplication of the IVC is observed in 0.2-3% of individuals. With this anomaly, the left IVC is usually smaller than the right, although they can be equal in size. The left IVC typically empties into the left renal vein (see the following image) and, subsequently, into the right IVC. Although the frequency of duplication is low, demonstration of the anomaly is important when determining the position for IVC filter placement. When the IVC is duplicated, filter placement in the right IVC may not be adequate to prevent PE, especially in the presence of a left lower-extremity DVT. Placement of a filter in both the left and right IVCs is often required for true PE prophylaxis in the patient with a duplicated IVC. The filter may also be placed suprarenally.
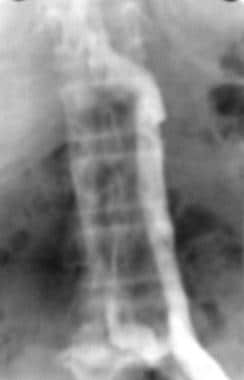 Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates duplication of the inferior vena cava. The left-sided inferior vena cava drains into the left renal vein.
Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates duplication of the inferior vena cava. The left-sided inferior vena cava drains into the left renal vein.
Transposition of the IVC
Transposition of the IVC is observed in 0.2-0.5% of individuals. A left-sided IVC drains into the left renal vein and crosses to the right of the spine. It then continues cranially in the normal position. The left renal and suprarenal veins empty directly into the left IVC; the right gonadal and suprarenal veins drain into the right renal vein and, subsequently, into the normal right-sided prerenal division of the left-sided IVC.
Circumaortic and retroaortic left renal vein
The incidence of circumaortic renal veins has been reported to be as high as 8.7% (see the image below). Patients with circumaortic or multiple renal veins may have a large hilar communication, which provides an alternative conduit for emboli. In the presence of a circumaortic renal vein, a filter immediately inferior to the main left renal vein may not represent adequate prophylaxis because of the alternative conduit; therefore, filters should be positioned inferior to the circumaortic renal vein. A retroaortic left renal vein is more common but does not impact filter placement, because it does not represent a potential conduit for thrombus arising from the lower extremities.
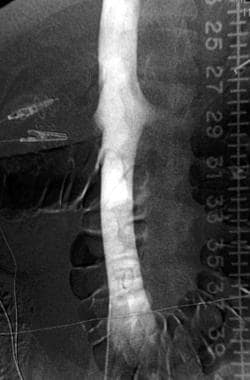 Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates a circumaortic left renal vein.
Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates a circumaortic left renal vein.
Preprocedural evaluations, assessing DVT and PE
Candidates for IVC filter placement are typically evaluated for DVT and/or PE. Imaging methods used to document lower-extremity DVT include ultrasonography (US) and peripheral venography. PE is usually diagnosed by nuclear medicine ventilation-perfusion (V/Q) scans.
Although pulmonary arteriography remains the criterion standard for confirmation of diagnosis, an increasing number of centers are using multichannel detector-row helical (MDH) computed tomography (CT) to evaluate patients in whom PE is suspected. Alternatively, magnetic resonance angiography (MRA) of the pulmonary vasculature can also be performed.
Laboratory assessment
Laboratory assessment typically includes the following:
-
Coagulation profile (including platelet count)
-
Prothrombin time (PT)
-
Activated partial thromboplastin time (aPTT)
-
Renal function with serum blood urea nitrogen (BUN) and creatinine levels
If a filter is to be placed in a patient who is receiving anticoagulation therapy, the medications are usually discontinued before the procedure. Discontinuing heparin on call is usually adequate, although some physicians prefer to discontinue heparin for 2-4 hours before the procedure.
Gray et al described the safety of venous interventional procedures, including pulmonary angiography and filter placement, in 100 patients receiving anticoagulation. [103] Of these patients, 87 had prolonged bleeding times. [103]
Assessing the IVC
The caliber of the IVC varies in accordance with the intravascular volume, the phases of respiration, and cardiac function. The mean diameter of the IVC is 19-20 mm; approximately 3% of patients have a megacava, in which the diameter is greater than 28 mm.
Imaging and cavography have been used to visualize the IVC for its size, its configuration, and any anatomic variation. Imaging is also used to evaluate the presence and extent of bland and tumor thrombus within the IVC.
Ultrasonography
The usefulness of US sometimes can be limited by overlying bowel gas and a large body habitus. This imaging modality is used more commonly in the evaluation of the common femoral veins or the internal jugular veins to assess patency before puncture and for guidance during venous access. More recently, intravascular US (IVUS) has been used as an imaging method before and during filter placement. [104]
CT scanning and MRI
CT scanning and MRI have been used to visualize the size, configuration, and anatomic variants of the IVC.
Inferior vena cavography
Currently, cavography is the modality used most commonly to assess the IVC, usually before filter placement. Access to the IVC is typically achieved via a left or right femoral vein or via the right internal jugular vein. The right femoral vein is the most common approach, accounting for 69% in a series reported by Athanasoulis et al. [5] US may be used to guide the puncture. After access into the venous system is gained, a pigtail catheter is typically positioned near the confluence of the common iliac veins.
Alternatively, catheter placement in the left common iliac vein enables visualization of both a normal IVC and a duplicated IVC, which is a normal variation of a double IVC.
In patients at intermediate risk for an adverse reaction to the contrast material and in patients with compromised renal function, cavography can be performed with carbon dioxide.
Various methods can be used to determine the diameter of the IVC, especially when digital subtraction angiography is performed. These include placement of a ruler with metallic markers along the left side of the patient's body and the use of marker catheters or guidewires.
In most centers, a single frontal projection is used for measurement, but biplane studies can also be used during the procedure.
More recently, investigators have advocated the use of preprocedural cross-sectional imaging for a more accurate assessment of the caval diameter.
Complications
Postprocedure complications may be related to jugular puncture or to the inferior vena cava (IVC) filter itself.
Complications related to jugular puncture
The following are complications associated with jugular puncture:
-
Pneumothorax (incidence very low with ultrasonographic guidance) [105]
-
Access site thrombosis (2%)
-
Persistent bleeding from insertion site
-
Pulmonary embolism (PE) (if passage was through a thrombosed vein)
Complications related to filter
The following are complications associated with IVC filters:
-
IVC trauma
-
Penetration of the caval wall by the filter legs (may rarely lead to retroperitoneal hematoma or bowel injury)
-
Filter migration (< 1%; may be caused by trapped guidewires)
-
Filter fracture (< 1%)
-
Filter infection (< 1%)
-
Caval thrombosis (5%)
-
Death (30-d mortality, < 1%)
Guidelines for Use of IVC Filters in Treatment of Venous Thromboembolism (SIR, 2020)
The guidelines on the use of inferior vena cava (IVC) filters in the treatment of patients with venous thromboembolism (VTE; ie, deep vein thrombosis [DVT] or pulmonary embolism [PE]) were published in October 2020 by the Society of Interventional Radiology (SIR), in collaboration with the American College of Cardiology (ACC), the American College of Chest Physicians (ACCP), the American College of Surgeons (ACS) Committee on Trauma, the American Heart Association (AHA), the Society for Vascular Surgery (SVS), and the Society for Vascular Medicine (SVM). [107]
In patients with acute PE who have a contraindication for anticoagulation therapy, consideration of IVC filter placement is suggested on the basis of various clinical risk factors.
In patients with acute DVT without PE who have a contraindication for anticoagulation therapy, consideration of IVC filter placement is suggested on the basis of various clinical risk factors.
In patients undergoing anticoagulation for acute VTE in whom a contraindication for anticoagulation develops, consideration of an IVC filter is suggested if there is ongoing significant clinical risk for PE.
In patients undergoing extended anticoagulation for VTE who have completed the acute phase of treatment and in whom a contraindication to anticoagulation develops, it is suggested that an IVC filter not be placed, with rare exceptions.
In patients receiving therapeutic anticoagulation for VTE who experience recurrent VTE, it is suggested that an IVC filter not be placed, with few exceptions. Reasons for anticoagulation failure should always be addressed.
In patients with acute VTE who are being treated with therapeutic anticoagulation, routine placement of an IVC filter is not recommended.
In patients with acute PE who are receiving advanced therapies, consideration of IVC filter placement is suggested only in select patients.
In patients with DVT who are receiving advanced therapies, consideration of IVC filter placement is suggested only in select patients.
In trauma patients without known acute VTE, it is recommended that routine placement of IVC filters for primary VTE prophylaxis not be performed.
In patients without known acute VTE who are undergoing major surgery, it is suggested that routine placement of IVC filters not be performed.
In patients with indwelling IVC filters who have no other indication for anticoagulation, no recommendation can be made for or against anticoagulation.
In patients with indwelling retrievable/convertible IVC filters whose risk of PE has been mitigated or who are no longer at risk for PE, it is suggested that filters be routinely removed or converted unless risk outweighs benefit.
In patients with indwelling permanent IVC filters whose risk of PE has been mitigated or who are no longer at risk for PE, it is suggested that filter removal not be routinely performed.
In patients with complications attributed to indwelling IVC filters, consideration of filter removal is suggested after weighing of filter- versus procedure-related risks and assessment of the likelihood that filter removal will alleviate the complications.
In patients who have an IVC filter, the use of a structured follow-up program is recommended to increase retrieval rates and detect complications.
In patients in whom IVC filter removal is planned, routine preprocedural imaging of the filter and the use of laboratory studies are not suggested, except in select situations.
In patients undergoing filter retrieval whose filter could not be removed with standard techniques, attempted removal with advanced techniques is suggested if appropriate and if the expertise is available, after reevaluation of risks and benefits.
In patients undergoing IVC filter placement, no recommendation can be made for or against any specific placement technique.
Venous Thromboembolism Clinical Practice Guidelines (ASH, 2020)
The American Society of Hematology (ASH) released their updated recommendations on the management of venous thromboembolism (VTE) (deep vein thrombosis [DVT] and pulmonary embolism [PE]) in October 2020. [106] Select recommendations are outlined below.
Strong Recommendations
For patients with PE and hemodynamic compromise, it is recommended that thrombolytic therapy followed by anticoagulation be used over anticoagulation alone.
For patients with DVT and/or PE who have completed primary treatment and will continue vitamin K antagonist (VKA) therapy as secondary prevention, it is recommended that an international normalized ratio (INR) range of 2.0 to 3.0 be used over a lower INR range (eg, 1.5-1.9).
For patients with a recurrent unprovoked DVT and/or PE, indefinite antithrombotic therapy is recommended over stopping anticoagulation after completion of primary treatment.
Conditional Recommendations
Initial management
For patients with DVT and/or PE, the ASH guideline panel suggests using direct oral anticoagulants (DOACs) over VKAs. No single DOAC is suggested over another.
In most patients with proximal DVT, anticoagulation therapy alone is suggested over thrombolytic therapy in addition to anticoagulation.
For patients with PE with echocardiography and/or biomarkers that are compatible with right ventricular dysfunction but without hemodynamic compromise (submassive PE), anticoagulation alone is suggested over the routine use of thrombolysis in addition to anticoagulation.
For patients with extensive DVT in whom thrombolysis is considered appropriate, the ASH guideline panel suggests using catheter-directed thrombolysis over systemic thrombolysis.
For patients with PE in whom thrombolysis is considered appropriate, systemic thrombolysis is suggested over catheter-directed thrombolysis.
For patients with proximal DVT and significant preexisting cardiopulmonary disease, as well as for patients with PE and hemodynamic compromise, use of anticoagulation alone is suggested rather than anticoagulation plus insertion of an inferior vena cava (IVC) filter.
Primary treatment
For primary treatment of patients with DVT and/or PE, whether provoked by a transient risk factor or by a chronic risk factor or unprovoked, using a shorter course of anticoagulation for primary treatment (3-6 months) is suggested over a longer course of anticoagulation for primary treatment (6-12 months).
Secondary prevention
To guide the duration of anticoagulation for patients with unprovoked DVT and/or PE, the ASH guideline panel suggests against routine use of prognostic scores, D-dimer testing, or ultrasonography to detect residual vein thrombosis.
Indefinite antithrombotic therapy is suggested over anticoagulation cessation after completion of primary treatment for the following:
-
Patients with DVT and/or PE provoked by a chronic risk factor
-
Patients with unprovoked DVT and/or PE
For patients with DVT and/or PE who have completed primary treatment and will continue to receive secondary prevention, use of anticoagulation is suggested over aspirin.
For patients with DVT and/or PE who have completed primary treatment and will continue with a DOAC for secondary prevention, the ASH guideline panel suggests using a standard-dose DOAC or a lower-dose DOAC.
Recurrent events
For patients with breakthrough DVT and/or PE during therapeutic VKA treatment, the ASH guideline panel suggests using low-molecular-weight heparin (LMWH) over DOAC therapy.
For patients who develop DVT and/or PE provoked by a transient risk factor and have a history of previous unprovoked VTE or VTE provoked by a chronic risk factor, indefinite antithrombotic therapy is suggested over stopping anticoagulation after completing primary treatment.
For patients who develop DVT and/or PE provoked by a transient risk factor and have a history of a previous VTE also provoked by a transient risk factor, anticoagulation cessation after completion of primary treatment is suggested over indefinite antithrombotic therapy.
Other
For patients with DVT and/or PE with stable cardiovascular disease (CVD) who initiate anticoagulation and were previously taking aspirin for cardiovascular risk modification, it is suggested that aspirin be suspended over continuing it for the duration of anticoagulation therapy.
For patients with DVT, with or without an increased risk for postthrombotic syndrome (PTS), the ASH guideline panel suggests against the routine use of compression stockings.
Additional Resources
For more information, please go to Venous Thromboembolism (VTE), Deep Venous Thrombosis (DVT), and Pulmonary Embolism (PE).
For more Clinical Practice Guidelines, please go to Guidelines.
Questions & Answers
Overview
What are inferior vena cava (IVC) filters?
What are the available types of permanent inferior vena cava (IVC) filters?
What are the available types of retrievable inferior vena cava (IVC) filters?
What is a Günther Tulip inferior vena cava (IVC) filter?
What are the properties and capabilities of inferior vena cava (IVC) filters?
Which technical considerations impact the approach to inferior vena cava (IVC) filter placement?
What is a Mobin-Uddin umbrella inferior vena cava (IVC) filter?
What is a Greenfield inferior vena cava (IVC) filter?
What is a titanium Greenfield inferior vena cava (IVC) filter?
What is a percutaneous stainless steel Greenfield inferior vena cava (IVC) filter?
What is a Bird's Nest inferior vena cava (IVC) filter?
What is a Vena Tech LGM inferior vena cava (IVC) filter?
What is a Vena Tech LP inferior vena cava (IVC) filter?
What is a Simon nitinol inferior vena cava (IVC) filter?
What is a TrapEase inferior vena cava (IVC) filter?
What is a SafeFlo inferior vena cava (IVC) filter?
What are retrievable inferior vena cava (IVC) filters?
What is a recovery nitinol inferior vena cava (IVC) filter?
What are G2 and G2 Xinferior vena cava (IVC) filters?
What is a OptEase inferior vena cava (IVC) filter?
What is a Celect inferior vena cava (IVC) filter?
What is an ALN inferior vena cava (IVC) filter?
What is an Option inferior vena cava (IVC) filter?
What is the most frequent indication for inferior vena cava (IVC) filters?
When is an inferior vena cava (IVC) filter indicated in a patient with new-onset DVT or PE?
When is an inferior vena cava (IVC) filter indicated for PE prophylaxis?
When is an inferior vena cava (IVC) filter contraindicated?
What vascular anatomy understanding is needed to place an inferior vena cava (IVC) filter?
Which anatomic variations may alter the placement of an inferior vena cava (IVC) filter?
What is included in the preprocedure assessment for inferior vena cava (IVC) filter candidates?
What are the types of possible complications from an inferior vena cava (IVC) filter placement?
What are the possible jugular complications related to an inferior vena cava (IVC) filter placement?
What are the possible complications associated with an inferior vena cava (IVC) filter?
Which organizations have issued recommendations for the management of venous thromboembolism (VTE)?
-
Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates an inferior vena cava of normal diameter without thrombus. In addition, the inflow from the renal veins can be seen at the level of the L1 vertebral body.
-
Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates duplication of the inferior vena cava. The left-sided inferior vena cava drains into the left renal vein.
-
Inferior Vena Cava Filters. Anteroposterior image from an inferior venacavographic examination demonstrates a circumaortic left renal vein.
-
Inferior Vena Cava Filters. Bird's nest filter.
-
Inferior Vena Cava Filters. Greenfield filter.
-
Inferior Vena Cava Filters. Simon nitinol filter.
-
Inferior Vena Cava Filters. TrapEase filter.