Practice Essentials
Respiratory distress syndrome (RDS) of the newborn is an acute lung disease caused by surfactant deficiency, which leads to alveolar collapse and noncompliant lungs. Neonatal RDS arises at or shortly after birth (< 24 hr) and increases in severity during the first 48 hours of life. Previously known as hyaline membrane disease, this condition is primarily seen in premature infants younger than 32 weeks’ gestation. The incidence of RDS is inversely proportional to the gestational age of the infant, with more severe disease in the smaller and more premature neonates (92% of neonates born at 24-25 weeks, 88% at 26-27 weeks, 76% at 28-29 weeks, 57% at 30-31 weeks). RDS is usually diagnosed with a combination of clinical signs and/or symptoms, chest radiographic findings, and arterial blood gas results. A normal film at 6 hours of life excludes the diagnosis of RDS. Lung ultrasound score (LUS) has been described in the early phases of neonatal RDS, but LUS may potentially miss pneumothorax, pneumomediastinum, or pneumopericardium; therefore, chest radiography remains necessary for suspected neonatal RDS. [1, 2, 3, 4, 5, 6, 7]
(Radiographic features of RDS are seen in the images below.)
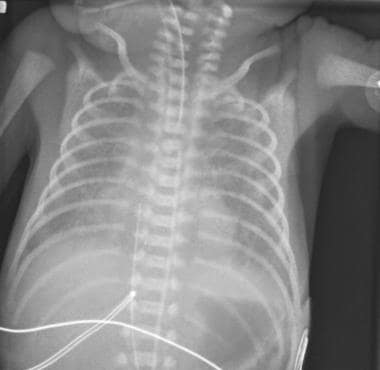 Classic respiratory distress syndrome (RDS). Bell-shaped thorax is due to generalized underaeration. Lung volume is reduced, the lung parenchyma has a fine granular pattern, and peripherally extending air bronchograms are present.
Classic respiratory distress syndrome (RDS). Bell-shaped thorax is due to generalized underaeration. Lung volume is reduced, the lung parenchyma has a fine granular pattern, and peripherally extending air bronchograms are present.
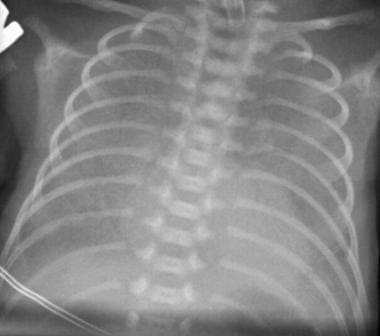 Moderately severe respiratory distress syndrome (RDS). The reticulogranular pattern is more prominent and uniformly distributed than usual. The lungs are hypoaerated. Increased air bronchograms are observed.
Moderately severe respiratory distress syndrome (RDS). The reticulogranular pattern is more prominent and uniformly distributed than usual. The lungs are hypoaerated. Increased air bronchograms are observed.
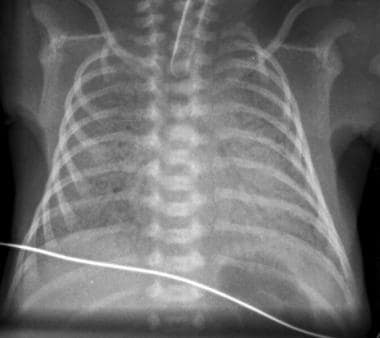 Severe respiratory distress syndrome (RDS). Reticulogranular opacities are present throughout both lungs, with prominent air bronchograms and total obscuration of the cardiac silhouette. Cystic areas in the right lung may represent dilated alveoli or early pulmonary interstitial emphysema (PIE).
Severe respiratory distress syndrome (RDS). Reticulogranular opacities are present throughout both lungs, with prominent air bronchograms and total obscuration of the cardiac silhouette. Cystic areas in the right lung may represent dilated alveoli or early pulmonary interstitial emphysema (PIE).
The incidence and severity of RDS are inversely related to gestational age. RDS is the most common cause of respiratory failure during the first days after birth. In addition to prematurity, other factors contributing to the development of RDS are maternal diabetes, cesarean delivery without preceding labor, [8] being the second born of twins, perinatal asphyxia, perinatal infection, and patent ductus arteriosus. [9, 10, 11]
Complications of RDS are numerous, both acute and chronic. [12] Infants with RDS are at risk of developing alveolar rupture and pulmonary interstitial emphysema, infection, intracranial hemorrhage, chronic lung disease (bronchopulmonary dysplasia), retinopathy of prematurity, neurologic impairment, and sudden death.
The outcome of patients with RDS has improved with the increased use of antenatal steroids to improve pulmonary maturity, early postnatal surfactant therapy to replace surfactant deficiency, and gentle techniques of ventilation to reduce barotrauma to the immature lungs.
A Cochrane meta-analysis showed that antenatal corticosteroid therapy in women at risk of preterm delivery reduced the incidence of RDS, neonatal death, and intraventricular hemorrhage. [13]
Meta-analyses have confirmed that exogenous surfactant treatment decreases overall morbidity and mortality in preterm newborns with RDS. A study by Soll et al demonstrated that multiple doses of animal-derived surfactant extract provided greater improvement than single-dose therapy with regard to oxygenation and ventilatory requirements, reduced risk of pneumothorax, and improved survival. [12] Efforts are ongoing to identify the optimal delivery method and dosages. [11]
In the delivery room, nasal continuous positive airway pressure (CPAP) is often used in spontaneously breathing premature infants immediately after birth as a potential alternative to immediate intubation and surfactant replacement to minimize the severity of bronchopulmonary dysplasia (BPD). Several centers have reported success with the use of nasal bubble CPAP to decrease the complications associated with intubation and mechanical ventilation. By avoiding intubation, lung injury may be diminished. [14] The European Guidelines for the Management of RDS recommends the use of CPAP at birth for all at-risk neonates born at less than 30 weeks' gestation who do not need immediate intubation. [15]
CPAP is also used as an adjunct therapy given after surfactant therapy and helps to prevent atelectasis and apnea.
Lahra et al found that maternal and fetal intrauterine inflammatory responses (chorioamnionitis and umbilical vasculitis) are protective for RDS. In this study, chorioamnionitis with umbilical vasculitis was found to provide a markedly greater reduction of RDS than the presence of chorioamnionitis alone. [16]
Radiography
In respiratory distress syndrome (RDS), the classic chest radiographic findings consist of pronounced hypoaeration, bilateral fine granular opacities in the pulmonary parenchyma, and peripherally extending air bronchograms.
The radiologic spectrum of RDS ranges from mild to severe and is generally correlated with the severity of the clinical findings. In the early stages of the disease, notable air bronchograms are lacking, because the major bronchi lie in the more anterior portions of the lungs and because alveolar atelectasis tends to involve the dependent areas of the lungs, which are posterior in recumbent infants. However, a bubble appearance, which represents overdistended bronchioles and alveolar ducts, can be observed. (See the image below.)
 Classic respiratory distress syndrome (RDS). Bell-shaped thorax is due to generalized underaeration. Lung volume is reduced, the lung parenchyma has a fine granular pattern, and peripherally extending air bronchograms are present.
Classic respiratory distress syndrome (RDS). Bell-shaped thorax is due to generalized underaeration. Lung volume is reduced, the lung parenchyma has a fine granular pattern, and peripherally extending air bronchograms are present.
Radiologic spectrum and course
The appearance of granular opacities is the result of superimposition of multiple acinar nodules caused by atelectatic alveoli and interstitial fluid. The development of air bronchograms depends on the coalescence of areas of acinar atelectasis around aerated bronchi and bronchioles. In nonintubated infants, cephalic doming of the diaphragms and hypoaeration are observed.
In infants with mild-to-moderate RDS, hypoaeration and fine granular opacities persist for 3-5 days following the administration of surfactant. Clearing from the peripheral to the central areas and from the upper lobe to the lower lobe begins at the end of the first week.
(See the image below.)
 Moderately severe respiratory distress syndrome (RDS). The reticulogranular pattern is more prominent and uniformly distributed than usual. The lungs are hypoaerated. Increased air bronchograms are observed.
Moderately severe respiratory distress syndrome (RDS). The reticulogranular pattern is more prominent and uniformly distributed than usual. The lungs are hypoaerated. Increased air bronchograms are observed.
As RDS progresses, the reticulogranular pattern becomes prominent due to coalescence of the small atelectatic areas. This coalescence leads to larger areas of increased lung opacity. As the anterior portions of the lung become involved with microatelectasis, the granularity becomes uniformly distributed, and air bronchograms can be seen. (See the image below.)
 Severe respiratory distress syndrome (RDS). Reticulogranular opacities are present throughout both lungs, with prominent air bronchograms and total obscuration of the cardiac silhouette. Cystic areas in the right lung may represent dilated alveoli or early pulmonary interstitial emphysema (PIE).
Severe respiratory distress syndrome (RDS). Reticulogranular opacities are present throughout both lungs, with prominent air bronchograms and total obscuration of the cardiac silhouette. Cystic areas in the right lung may represent dilated alveoli or early pulmonary interstitial emphysema (PIE).
With increasing severity of disease and worsening hypoaeration, progressive opacification of the anterior portions of the lungs cause obscuration of the cardiac silhouette and the formation of prominent air bronchograms. With severe disease, the lungs appear opaque and display prominent air bronchograms, with total obscuration of the cardiomediastinal silhouette. This type of severe and progressive RDS often leads to death, usually within 72 hours.
RDS during treatment
The radiographic findings of RDS depend on the timing of the administration of surfactant.
Because the surfactant is not evenly distributed throughout the lungs, areas of improving lung alternating with areas of unchanged RDS is common. This uneven distribution leads to a radiographic appearance similar to that of other entities, such as neonatal pneumonia and meconium aspiration syndrome. The clearing is sometimes irregular, creating a cystic appearance. Relapse may occur after initial improvement.
Infants who are being ventilated with intermittent positive pressure with positive end-expiratory pressure may have well-aerated lungs without air bronchograms. Infants with severe disease may be unable to expand their lungs; they have total opaque radiographs.
Because infants with RDS are usually hypoxic, the ductus arteriosus may remain patent. Early in the disease, shunting is from right to left. By the end of the first week, shunting becomes left to right as pulmonary artery pressure decreases because of the increased compliance of the healing lungs. Interstitial pulmonary edema may develop. Therefore, when the granular pattern of hyaline membrane disease changes to a homogeneously opaque appearance, pulmonary edema due to patent ductus arteriosus or early chronic pulmonary changes should be suspected.
Complications
Early
With positive-pressure ventilation, the lungs’ opacity decreases, and they appear radiographically improved. However, the positive pressure required to aerate the lungs can disrupt the epithelium, producing interstitial and alveolar edema. It can also cause the dissection of air into the interlobar septae and their lymphatics, producing pulmonary interstitial emphysema (PIE), which has the appearance of tortuous, 1- to 4-mm linear lucencies that are relatively uniform in size. These radiate outward from the hilar regions. The lucencies do not empty on expiration and extend to the periphery of the lungs. (PIE is shown in the image below.)
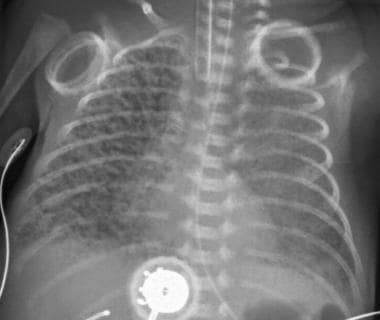 Complication of respiratory distress syndrome (RDS). After receiving ventilation therapy, this premature infant with RDS developed pulmonary interstitial emphysema (PIE) with discrete linear and cystic radiolucent air collections throughout the right lung.
Complication of respiratory distress syndrome (RDS). After receiving ventilation therapy, this premature infant with RDS developed pulmonary interstitial emphysema (PIE) with discrete linear and cystic radiolucent air collections throughout the right lung.
PIE can be symmetrical, asymmetrical, or localized to one portion of a lung. Peripheral PIE can produce subpleural blebs and ultimately rupture into pleural space to produce pneumothorax (usually tension pneumothorax, shown in the image below), or they can extend centrally to produce pneumomediastinum or pneumopericardium. Because infants are supine and because air rises to the highest point of the thorax, the pneumothorax is located paramediastinally, resulting in the sharp mediastinum sign, whereby the mediastinum/heart is sharply outlined by adjacent free air rather than aerated lung tissue.
A continuous diaphragm sign, which is caused by air in the mediastinum beneath the heart, may be seen with pneumomediastinum. When alveoli rupture, air may become localized and may coalesce in the parenchyma to produce pulmonary pseudocyst. In addition to parenchymal pseudocysts and PIE, alveolar rupture may allow air to enter the pulmonary venous system, leading to systemic air embolism with intravascular air.
Late
Late in the course of the disease, pulmonary edema, air leaks, or pulmonary hemorrhage can affect the radiographic appearance.
Radiographic findings in conditions mimicking RDS
Meconium aspiration syndrome (shown below) usually occurs in postterm infants, especially in those with meconium staining. Clinical symptoms usually appear 12-24 hours after birth. (In contrast, clinical symptoms of RDS always appear within the first few hours of life.)
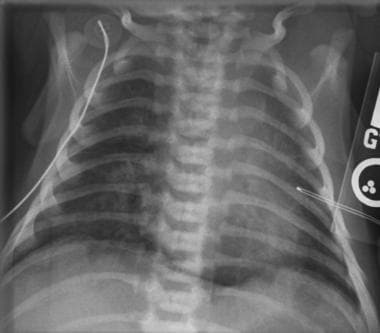 Meconium aspiration syndrome. Air trapping; diffuse, coarse nodular opacities; and areas of focal emphysema typical of meconium aspiration are different from diffuse the finely granular opacities seen in RDS. Lungs are usually hyperaerated. Image also shows pneumomediastinum with a continuous diaphragm sign caused by air in the mediastinum beneath the heart.
Meconium aspiration syndrome. Air trapping; diffuse, coarse nodular opacities; and areas of focal emphysema typical of meconium aspiration are different from diffuse the finely granular opacities seen in RDS. Lungs are usually hyperaerated. Image also shows pneumomediastinum with a continuous diaphragm sign caused by air in the mediastinum beneath the heart.
The most common radiographic features are hyperaeration and bilateral, diffuse, and grossly patchy areas of increased radiopacity. Pneumothorax in fetal aspiration syndrome is usually not tension pneumothorax; therefore, it often requires no specific therapy. In RDS, the lungs are hypoaerated, and the abnormal lung opacities due to atelectasis are finely granular. In addition, pneumothorax related to RDS is often under tension, and surgical intubation is required.
Transient tachypnea of the newborn (TTN), seen in the image below, usually occurs in term infants, usually after cesarean delivery. Clinical symptoms usually manifest within 6 hours of birth. Radiographic findings include increased or normal lung volume, with interstitial edema and pleural effusions. In RDS, bilateral reticular or granular parenchymal opacities are present for at least 3-4 days, whereas in transient tachypnea, these opacities are fleeting. Hypoaeration is typical of RDS, in contrast to the hyperaeration of transient tachypnea.
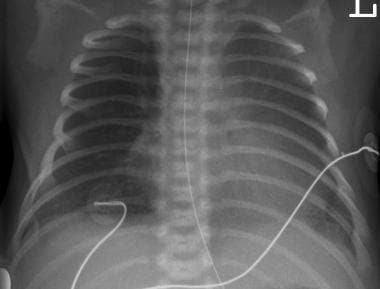 Transient tachypnea of the newborn (TTN). Hyperaeration is typical of TTN, in contrast to the hypoaeration of respiratory distress syndrome (RDS). Bilateral reticulogranular densities are fleeting with TTN and disappear with ventilation, whereas these opacities are present for at least 3-4 days in RDS.
Transient tachypnea of the newborn (TTN). Hyperaeration is typical of TTN, in contrast to the hypoaeration of respiratory distress syndrome (RDS). Bilateral reticulogranular densities are fleeting with TTN and disappear with ventilation, whereas these opacities are present for at least 3-4 days in RDS.
Neonatal pneumonia is usually associated with premature rupture of membranes. Clinical symptoms appear less than 6 hours after birth. Radiographic findings include perihilar streaking. Neonatal pneumonia often produces hyperaeration of the lungs, but in general, the areas of pneumonia are focal rather than diffuse. Pleural effusions may be the only distinguishing feature; they are not a feature of uncomplicated RDS but are present in as many as two thirds of patients with pneumonia. Group B beta-hemolytic streptococcal pneumonia often occurs with RDS, or it can mimic the appearance of RDS. Hence, many neonatal units give antibiotics to all neonates with this condition until blood cultures are negative.
Differentiating RDS from diffuse pulmonary hemorrhage may be difficult. One feature that aids in the differential diagnosis is the identification of a pleural effusion. Pleural effusions are rare in RDS but are common in pulmonary hemorrhage.
If chest images in a premature infant show reticulogranular opacities, RDS can be diagnosed with 90% confidence.
Ultrasonography
The use of lung ultrasound in diagnosing neonatal respiratory distress syndrome (RDS) has shown great promise. A study by Liu et al has suggested that it can be an accurate and reliable modality that is also rapid, portable, and nonionizing. [17] In addition, a meta-analysis of 9 studies found a pooled sensitivity of 0.99 (CI: 0.92-1.00) and a specificity of 0.95 (CI: 0.87-0.98). [18] Other studies have supported these findings. [2, 3, 4, 5, 6, 7]
The many benefits of ultrasound include its ability to differentiate between the possible causes of respiratory failure using serial bedside images that do not expose the infant to ionizing radiation. [19]
Findings of lung consolidation, pleural-line abnormalities, lung pulse, bilateral “white lung” or alveolar interstitial syndrome, and A-line disappearance were found to be very sensitive and very specific when compared with conventional portable chest radiography. In addition, ultrasonography can be useful to diagnose or exclude a simultaneous or complicating pleural effusion. [20, 19]
Bedside lung ultrasonography was found to be superior to chest radiography in detecting consolidation and subpleural atelectasis, but not in detecting pneumothorax, in 90 premature newborns with respiratory distress syndrome. Alveolo-interstitial syndrome and pleural-line abnormalities were detected in all cases in the initial assessment; patchy consolidation was detected in 34 cases; and white lung was detected in 80 cases. [21]
In 23 infants with respiratory distress syndrome, lung ultrasound (LUS) showed a sensitivity of 95.6%, specificity of 94.4%, positive predictive value of 91.6%, and negative predictive value of 97.1%. For transient tachypnea of the newborn in 30 infants, lung ultrasound showed a sensitivity of 93.3%, specificity of 96.5%, positive predictive value of 96.5%, and negative predictive value of 93.4%. [22]
Raimondi et al found that there was a significant correlation between LUS and the ratio of oxygen saturation to inspired oxygen throughout admission, increasing with gestational age. In infants 25 to 30 weeks' gestation, the LUS at 7 days of life predicted bronchopulmonary dysplasia with an area under the curve of 0.82 (95% confidence interval 0.71 to 93). [2]
Szymanski et al found that early post-birth LUS predicted ventilation requirements on day of life 3. [3] In a study, by Perri et al, of 56 newborns with a mean gestational age of 31 weeks and a mean birth weight of 1442 g, LUS scores showed higher AUC (area under curve) than x-ray scores in the early recognition of infants with RDS who required surfactant treatment. In addition, LUS had a higher sensitivity (86% vs 82%), higher specificity (88% vs 76%), better positive predictive value (83% vs 69%), and better negative predictive value (91% vs 87%). [5]
-
Classic respiratory distress syndrome (RDS). Bell-shaped thorax is due to generalized underaeration. Lung volume is reduced, the lung parenchyma has a fine granular pattern, and peripherally extending air bronchograms are present.
-
Moderately severe respiratory distress syndrome (RDS). The reticulogranular pattern is more prominent and uniformly distributed than usual. The lungs are hypoaerated. Increased air bronchograms are observed.
-
Severe respiratory distress syndrome (RDS). Reticulogranular opacities are present throughout both lungs, with prominent air bronchograms and total obscuration of the cardiac silhouette. Cystic areas in the right lung may represent dilated alveoli or early pulmonary interstitial emphysema (PIE).
-
Complication of respiratory distress syndrome (RDS). After receiving ventilation therapy, this premature infant with RDS developed pulmonary interstitial emphysema (PIE) with discrete linear and cystic radiolucent air collections throughout the right lung.
-
Complication of respiratory distress syndrome (RDS). Anteroposterior (AP) chest radiograph in a neonate with RDS shows a right tension pneumothorax with herniation of right upper lung across midline. Pneumomediastinum is also present.
-
Complication of ventilation therapy. Bronchopulmonary dysplasia. Anteroposterior (AP) chest radiograph in a 4-week-old premature infant with history of respiratory distress syndrome (RDS) and receiving mechanical ventilation shows moderate pulmonary hyperinflation, coarse interstitial opacities with a honeycomb appearance throughout both lungs, and atelectasis in the right upper lobe.
-
Transient tachypnea of the newborn (TTN). Hyperaeration is typical of TTN, in contrast to the hypoaeration of respiratory distress syndrome (RDS). Bilateral reticulogranular densities are fleeting with TTN and disappear with ventilation, whereas these opacities are present for at least 3-4 days in RDS.
-
Meconium aspiration syndrome. Air trapping; diffuse, coarse nodular opacities; and areas of focal emphysema typical of meconium aspiration are different from diffuse the finely granular opacities seen in RDS. Lungs are usually hyperaerated. Image also shows pneumomediastinum with a continuous diaphragm sign caused by air in the mediastinum beneath the heart.








