Practice Essentials
Metastasis to the brain is the most feared complication of systemic cancer and the most common intracranial tumor in adults. The incidence of brain metastasis is rising with the increase in survival of cancer patients. Cancer patients now live longer as a result of important advances in cancer diagnosis and management—particularly, the widespread use of magnetic resonance imaging (MRI) to detect small metastases. Approximately 40% of intracranial neoplasms are metastatic. Multiple, large autopsy series suggest that, in order of decreasing frequency, lung (40%), breast (20%), melanoma (10%), and enteric cancers (6%) are the most common primary tumors to metastasize to the brain. [1]
Brain metastasis affects up to one third of adults with cancer and carries a historically bleak prognosis. Despite advances in stereotactic radiosurgery (SRS), rates of in-field recurrence after SRS range from 10 to 25%. High rates of neurologic death have been reported after SRS failure, particularly for recurrences deep in the brain and surgically inaccessible. [2]
(See the image below.)
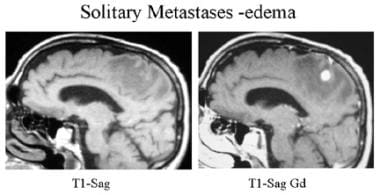 Sagittal T1-weighted precontrast and postcontrast MRI. A surgically proven, solitary right parietal metastasis is seen with a single, 1-cm, enhancing right parietal nodule and extensive surrounding edema typical of a metastasis.
Sagittal T1-weighted precontrast and postcontrast MRI. A surgically proven, solitary right parietal metastasis is seen with a single, 1-cm, enhancing right parietal nodule and extensive surrounding edema typical of a metastasis.
Brain metastases are an increasingly important cause of morbidity and mortality among cancer patients. Thus, brain metastasis presents a therapeutic challenge for the treating physician and is an emotionally and physically debilitating event for the patient. Early diagnosis and aggressive treatment of brain metastasis may result in remission of brain symptoms and may enhance the quality of the patient's life while prolonging survival.
The prognosis for patients with brain metastases is typically poor. Most available treatment is palliative; however, consideration should be given to prolonging good quality of life for patients by providing specific therapy to the brain. Evidence suggests that minimally invasive stereotactic radiosurgery (SRS) can be a safe treatment option for patients with up to 10 brain metastatic nodules. [3] The radiologist plays a primary role in management of cancer by providing accurate information on number, size, and locations of metastatic lesions, which is crucial for selecting the most appropriate treatment method for patients. [4]
Imaging modalities
Most patients with a known primary tumor undergo imaging studies when neurologic signs and symptoms develop. Magnetic resonance imaging (MRI) with contrast enhancement is the procedure of choice because MRI is more sensitive and specific than other imaging modalities in determining the presence, location, and numbers of metastases. [1] However, contrast-enhanced computed tomography (CT) scanning is used widely because of its accessibility and low cost. [5, 6, 7, 8, 9] In situations where MRI is not possible, contrast-enhanced CT can be helpful in detecting areas of neurovascular unit disruption and in defining the contrast-enhancing tumor burden for large areas of disease.
Even though it is less sensitive than MRI in tumor detection and characterization, non-contrast CT imaging is commonly performed as the first screening modality in emergent situations where a patient presents with new-onset neurologic symptoms, because it is highly sensitive in detecting the acute life-threatening complications (ie, hemorrhage, hydrocephalus, and herniation) of metastatic disease. [1]
With regard to screening for intracranial metastases, no consensus has been reached concerning when CT or MRI should be used for initial staging evaluation of a patient with cancer. However, brain MRI for patients with primary cancers that frequently metastasize to the brain (eg, bronchogenic carcinoma) is probably cost-effective. Numerous studies have shown that contrast-enhanced MRI detects 2 to 3 times as many lesions as contrast-enhanced CT, especially lesions that measure less than 5 mm in diameter. In addition, approximately 20% of patients with solitary metastatic lesions on CT show multiple lesions on MRI. The decision to perform imaging for patients with other cancers is made on the basis of the clinical evaluation.
In the presence of multiple cerebral metastases from an unknown primary source, a limited search for the primary tumor is of value; such a search includes chest radiography, breast examination and mammography, and abdominal ultrasonography. An extensive search for an occult malignancy is unrewarding. Surgery may be required when patients present with a solitary intracranial tumor or when a search for a possible primary tumor is needed.
Approximately one third of patients operated on for a single cerebral metastasis diagnosed with contrast-enhanced CT probably have more than 1 lesion. Contrast-enhanced MRI is more sensitive than CT in discerning the number of cerebral metastases.
Angiography is not used as a primary diagnostic procedure for metastatic disease. Rarely, preoperative angiography and embolization of large hypervascular metastases from renal and thyroid cancer may be useful. Angiography is useful for evaluating tumor vascularity in selected mesastatic lesions before biopsy is performed. Results of angiography are nonspecific for the diagnosis of metastases.
Radiography
Skull radiographs may detect multiple lytic or sclerotic deposits when the metastatic process involves the cranium. Lung and breast tumors are the primary malignancies that most commonly affect the skull. Multiple lytic lesions secondary to multiple myeloma tend to be uniformly small. Blastic metastases are seen in patients with primary prostate cancer or in patients who have undergone treatment for breast cancer. Calcifications are uncommon in metastases, but they do occur in primary adenocarcinoma, osteogenic sarcoma, and lung and breast carcinoma. Plain radiographs are not helpful in detecting metastatic disease of the brain.
Multiple lytic or blastic lesions are highly suggestive of a metastatic process. Solitary lesions must be differentiated from other pathologic processes affecting the skull vault.
Normal anatomic variants such as emissary vein, arachnoid granulation, and bone island may mimic a metastatic lesion in a patient known to have cancer. Use of CT with bone windows may eliminate false diagnoses.
Computed Tomography
Metastases frequently are multiple; they are seen at the junction of gray and white matter, usually with significant surrounding edema. [10, 11]
(See the images below.)
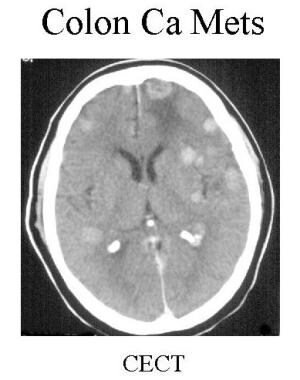 Contrast-enhanced CT shows multiple enhancing metastatic nodules at the gray-white junction in a patient with known colon cancer.
Contrast-enhanced CT shows multiple enhancing metastatic nodules at the gray-white junction in a patient with known colon cancer.
On noncontrast computed tomography (CT), the density of metastatic lesions may be less than, equal to, or greater than that of adjacent brain parenchyma. Most patterns are variable and are nondiagnostic, as shown in the images below.
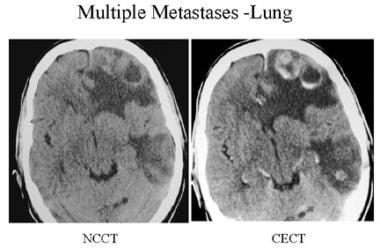 Precontrast- and postcontrast-enhanced CT shows multiple ring-enhancing lesions (thick, peripheral, ringlike) in the left hemisphere with prominent surrounding edema and mass effect in a patient with known lung cancer.
Precontrast- and postcontrast-enhanced CT shows multiple ring-enhancing lesions (thick, peripheral, ringlike) in the left hemisphere with prominent surrounding edema and mass effect in a patient with known lung cancer.
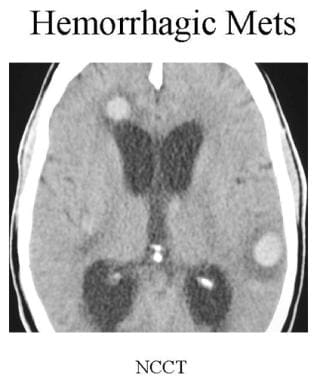 Noncontrast CT of a patient with lung cancer. Multiple high-density lesions are seen in both cerebral hemispheres, suggestive of hemorrhagic metastases.
Noncontrast CT of a patient with lung cancer. Multiple high-density lesions are seen in both cerebral hemispheres, suggestive of hemorrhagic metastases.
Noncontrast CT is performed to detect hemorrhage into metastases. Hyperdensity in a metastasis is more likely to involve hemorrhage than calcification.
(See the image below.)
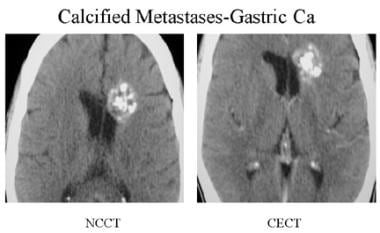 Precontrast- and postcontrast-enhanced CT scan of a patient with mucin-secreting adenocarcinoma of the stomach. Multiple calcified nodules are seen in the surgically proven metastasis.
Precontrast- and postcontrast-enhanced CT scan of a patient with mucin-secreting adenocarcinoma of the stomach. Multiple calcified nodules are seen in the surgically proven metastasis.
Intravenous (IV) administration of contrast material (30-40 g iodine) improves the diagnostic accuracy of CT. Most metastases are enhanced after a standard dose of IV contrast is given. Use of a higher dose of contrast (80-85 g iodine) along with delay of scanning by 1 to 3 hours after injection of the contrast agent leads to further increase in the detection of multiple metastases; such an approach is appropriate if MRI is not available.
Detection of additional metastases has important diagnostic and therapeutic implications. In cases in which there is no known primary cancer, if a solitary lesion is found on routine enhanced CT, the presence of an additional lesion may suggest a metastatic process, provided the solitary lesion is believed to be a primary lesion. In cases involving a solitary metastatic lesion of the brain, detection of an additional lesion may have a bearing on treatment; with multiple lesions, surgical treatment may be avoided in favor of chemotherapy, radiation therapy, or both.
Contrast-enhanced CT is effective in detecting major leptomeningeal spread. Contrast-enhancing subdural or epidural metastases may be seen, usually secondary to calvarial lesions. It is known that 5% of breast, lung, prostate, and renal cell neoplasms metastasize to the calvarium; of these, 15% extend into the subdural space.
(See the images below for contrast-enhanced CTs.)
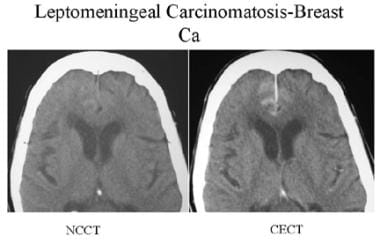 Precontrast- and postcontrast-enhanced CT of a patient with breast cancer. Linear enhancement in the anterior falx and medial sulci of both frontal lobes suggests leptomeningeal and dural carcinomatosis.
Precontrast- and postcontrast-enhanced CT of a patient with breast cancer. Linear enhancement in the anterior falx and medial sulci of both frontal lobes suggests leptomeningeal and dural carcinomatosis.
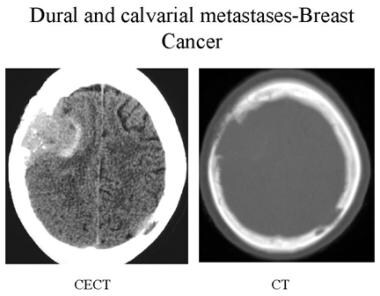 Contrast-enhanced CT and bone CT of a patient with breast cancer. Multiple irregular and aggressive lytic lesions are seen in the calvarium with dural metastases.
Contrast-enhanced CT and bone CT of a patient with breast cancer. Multiple irregular and aggressive lytic lesions are seen in the calvarium with dural metastases.
Degree of confidence
On findings of multiple, enhancing solid lesions at the gray matter–white matter junction and prominent surrounding edema in a patient with known primary cancer, metastases may be confidently diagnosed. Approximately 90% of patients with a history of cancer who present with a single supratentorial lesion have brain metastases.
Patients with multiple lesions are even more likely to have metastatic disease. Before undergoing definitive therapy, patients who are found to have a single metastasis on contrast-enhanced CT should undergo a contrast-enhanced MRI examination, if facilities for such an examination are available.
Routine cranial CT is useful for staging of cancer in the patient with non–small-cell lung cancer; cranial CT has a sensitivity of 92%, a specificity of 99%, and accuracy of 98% in detecting brain metastases. Contrast-enhanced CT is perhaps the best method by which to identify calvarial metastases. In studies comparing contrast-enhanced CT with contrast-enhanced MRI, in approximately 20% of patients with a single lesion evident on CT, multiple lesions were revealed on MRI. Most often, lesions missed on contrast-enhanced CT were smaller (< 2 cm in diameter) and appeared next to the bone in a frontotemporal location. Dural metastases may mimic meningioma.
Magnetic Resonance Imaging
Multiple lesions with marked vasogenic edema and mass effect are typically seen on magnetic resonance imaging (MRI) in patients with brain metastases, as shown in the images below. [7, 8, 9, 12, 13, 14, 15, 16, 17]
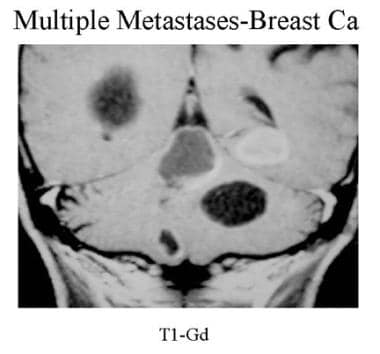 Coronal contrast-enhanced T1-weighted MRI. Multiple solid and cystic supratentorial and infratentorial mass lesions are seen in a patient with known breast cancer. Note the cystic, ring-enhancing, and solid patterns.
Coronal contrast-enhanced T1-weighted MRI. Multiple solid and cystic supratentorial and infratentorial mass lesions are seen in a patient with known breast cancer. Note the cystic, ring-enhancing, and solid patterns.
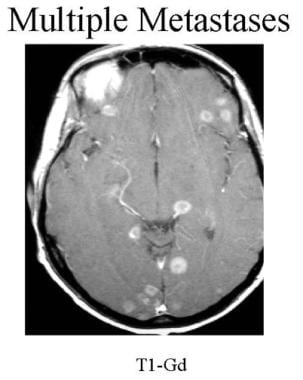 Axial contrast-enhanced T1-weighted MRI of a patient with known lung cancer. Multiple, small ring-enhancing lesions are seen in both hemispheres.
Axial contrast-enhanced T1-weighted MRI of a patient with known lung cancer. Multiple, small ring-enhancing lesions are seen in both hemispheres.
Lesions are isointense to mildly hypointense on T1-weighted images; they are hyperintense on T2-weighted images or on fluid attenuation inversion recovery.
Surrounding edema is relatively hypointense on fluid attenuation inversion recovery and on T1-weighted images; it is hyperintense on T2-weighted images.
Hemorrhagic metastases or melanoma lesions are hyperintense on T1-weighted images.
On T2-weighted images, mucinous adenocarcinoma may be hypointense because of calcification; hemorrhagic metastases may be hypointense as the result of chronic breakdown of blood products.
Following administration of a contrast agent, solid, nodular (see the first image below), or irregular ring patterns of enhancement are seen. Nonenhancing lesions (see the second image below) are less likely to signify metastases.
 Axial contrast-enhanced T1-weighted MRI of a patient with known lung cancer. Multiple, small ring-enhancing lesions are seen in both hemispheres.
Axial contrast-enhanced T1-weighted MRI of a patient with known lung cancer. Multiple, small ring-enhancing lesions are seen in both hemispheres.
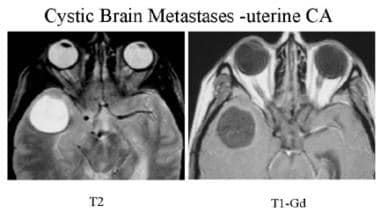 T2-weighted and postcontrast T1-weighted MRI of a patient with uterine cancer. Well-defined, nonenhancing, round lesion with fluid signal intensity is seen in the right temporal lobe, with prominent surrounding edema. This is a surgically proven cystic metastasis.
T2-weighted and postcontrast T1-weighted MRI of a patient with uterine cancer. Well-defined, nonenhancing, round lesion with fluid signal intensity is seen in the right temporal lobe, with prominent surrounding edema. This is a surgically proven cystic metastasis.
Contrast-enhanced MRI is the best method for detecting meningeal tumor seeding, which appears as abnormal dural enhancement. This is a nonspecific finding; however, in the appropriate clinical setting, it correlates with the presence of sheets of tumor cells affecting the meninges.
Approximately 30% of patients with metastatic disease to the central nervous system (CNS) present with a solitary brain parenchymal lesion; differentiating a solitary metastasis from a high-grade glioma is clinically important, as medical staging, surgical planning, and therapeutic decision-making are drastically different depending on the diagnosis. It is difficult to distinguish a primary glioma from an intercranial metastasis based on standard MRI. However, reports have described successful use of diffusion-weighted and perfusion-weighted imaging and proton-MR spectroscopy for differentiation of brain gliomas from solitary brain metastases. [1, 18]
High-resolution stereotactic MRI at the time of gamma knife surgery may reveal additional brain metastases, decreasing the incidence of, and lengthening the time to, distant recurrences. [19]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). [20] NSF/NFD has occurred in patients with moderate to end-stage renal disease after they were given a gadolinium-based contrast agent to enhance MRI or magnetic resonance angiography (MRA) scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Degree of confidence
Gadolinium-enhanced MRI is superior to contrast-enhanced CT in the diagnosis of brain metastases. Gadolinium-enhanced MRI offers the following advantages:
-
Utility for detecting smaller lesions
-
Better soft tissue contrast
-
Relatively stronger enhancement with paramagnetic contrast agents
-
No bone artifacts in the images
-
Fewer partial-volume effects, particularly for lesions adjacent to bones
-
Direct multiplanar imaging
Use of magnetization transfer with single-dose gadolinium administration is roughly equivalent to triple-dose, postcontrast, spin-echo imaging for detecting lesions and lesion conspicuity (defined as the ratio between lesion contrast and surround complexity). Although observer error is the most significant cause of misdiagnosis, tumor characteristics such as lesion size, conspicuity, and location are also crucial in this context. [21]
It has been shown that treatment with dexamethasone leads to reduced evidence on MRI of peritumoral edema and, occasionally, to lessening of the extent of contrast enhancement. If a lesion is found and a definitive diagnosis cannot be established, biopsy should be performed.
High-dose gadoteridol is better able to detect additional smaller lesions when compared with routine-dose gadopentetate dimeglumine. Detection of additional lesions is important when one is considering surgical treatment of a solitary lesion. Magnetization transfer done with routine-dose gadolinium contrast is closely comparable to the high-dose technique.
Differentiation between radiation-induced changes and tumor recurrence is a major pitfall of MRI, which can be overcome by the use of positron emission tomography (PET). Although amino acid PET tracers have provided several advantages over fluorodeoxyglucose in neuro-oncology, studies comparing these 2 types of radiopharmaceuticals in previously irradiated brain metastases are lacking. [22]
Evaluation of brain metastases generally requires postcontrast MRI, but some patients have contraindications to contrast medium administration. In a retrospective analysis of MRI data from patients with brain metastases, Liheng et al investigated the value of MRI diffusion tensor imaging (DTI) for detection of metastatic brain tumor. Results showed that DTI may detect more brain metastatic lesions than T1- and T2-weighted imaging. They speculated that the DTI original image can be used as an alternative examination for patients with contraindications to contrast-enhanced MRI. [23]
False positives/negatives
On imaging, dural metastases (see the first image below) may resemble meningiomas. Leptomeningeal carcinomatosis (see the second image below) may resemble chronic meningitis; however, an appropriate history or detection of primary cancer may be sufficient for establishing the diagnosis. Leptomeningeal enhancement may occur after administration of radiation or following extra-axial hemorrhage; it may also be noted below a craniotomy site. Single or multiple ring-enhancing lesions with edema may resemble infectious processes. Solitary lesions resemble primary brain tumors.
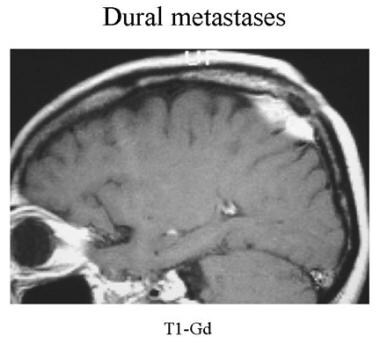 Sagittal contrast-enhanced MRI of a patient with known prostate cancer. The solitary enhancing right parietal extra-axial lesion is a surgically proven dural metastasis.
Sagittal contrast-enhanced MRI of a patient with known prostate cancer. The solitary enhancing right parietal extra-axial lesion is a surgically proven dural metastasis.
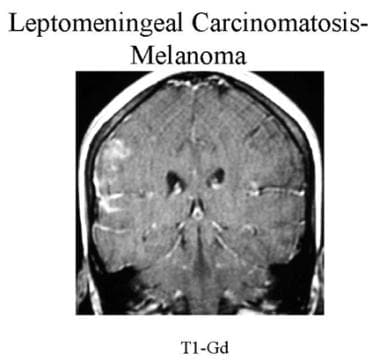 Coronal contrast-enhanced MRI of a patient with known melanoma. Note linear enhancement in the sulci and brain surface of the right frontal and temporal lobes suggestive of leptomeningeal carcinomatosis. The cancer was proven by cerebrospinal fluid cytology.
Coronal contrast-enhanced MRI of a patient with known melanoma. Note linear enhancement in the sulci and brain surface of the right frontal and temporal lobes suggestive of leptomeningeal carcinomatosis. The cancer was proven by cerebrospinal fluid cytology.
Nuclear Imaging
Nuclear medicine studies are not employed routinely as primary imaging techniques for detecting intracranial metastatic disease. Typical findings include multiple intracerebral areas of increased activity. The standard isotope used is technetium-99m (99mTc). On isotope whole-body bone scans, calvarial metastases may appear as multiple focal areas of increased activity. With whole-body 18-fluorodeoxyglucose (FDG) positron emission tomography (PET) used in cancer staging, intracerebral metastases may appear as areas of increased metabolism. [24, 25, 26, 27, 28]
Historically, radionuclide imaging was used to detect intracerebral metastases in approximately 90% of patients, but findings were nonspecific. Neoplasm, inflammation, vascularity, or trauma may cause abnormal uptake. FDG-PET has been reported to detect approximately two thirds of brain metastases resulting from systemic cancer. [29, 30]
PET imaging is being used increasingly to supplement MRI in clinical management of brain tumors, with different PET radiotracers targeting various biological processes to depict brain tumors and provide metabolic and biologic information. Radiolabeled amino acids such as 18F-fluorodihydroxyphenylalanine (18F-DOPA) and 18F-fluoroethyltyrosine (18F-FET) are used to identify gliomas. Meningiomas can be seen with high contrast when radiolabeled ligands of somatostatin receptors are used.ref31}
Future Directions
Melanoma brain metastasis (MBM) frequently occurs in patients with advanced melanoma. However, our understanding of the underlying salient biology is limited. Biermann and associates in 2022 sought to provide comprehensive insights into MBM biology by performing single-cell/nucleus RNA-seq in 22 treatment-naive MBMs and 10 extracranial melanoma metastases with matched spatial single-cell transcriptomics and T-cell receptor-seq. They discovered that cancer cells from MBM were more chromosomally unstable, adopted a neuron-like cell state, and were enriched for spatially variably expressed metabolic pathways. This work serves as a foundational resource for further exploration. [31]
Amino acid positron emission tomography (PET) has been used in the management of brain metastases, but histopathologic correlates of PET findings remain poorly understood. In 2021, Meyer and associates investigated the relationship of O-(2-18F-fluoroethyl-L-tyrosine (18F-FET) PET, magnetic resonance imaging (MRI), and histology in evaluating brain metastases. They found that 18F-FET PET had higher specificity than MRI (66% vs 56%) and increased sensitivity for tumor from 73% to 93% when combined with MRI. Researchers concluded that 18F-FET PET can be a valuable tool in the management of brain metastases. When performing biopsies, one should aim for PET hotspots to increase the chance for retrieval of samples with high tumor cell concentrations. Multiple biopsies should be performed to account for intratumor heterogeneity. Study authors proposed that PET could be useful for differentiating treatment-related changes (eg, radiation necrosis) from tumor recurrence. [32]
Metastases are the most common intracranial malignancies; complete resection can provide relief of neurologic symptoms and can reduce recurrence. In this prospective cohort study, authors evaluated intraoperative imaging and clinical outcomes of treatment using second-window indocyanine green (SWIG). They reported clinical benefits of the SWIG technique in surgery for patients with brain metastases. Specifically, this technique allows for dose-dependent, transdural localization of neoplasms and improved sensitivity in detection of neoplastic margins. Researchers concluded that postresection residual fluorescence can be a powerful tool for evaluating the extent of resection when used in conjunction with MRI, thereby guiding decisions on management of brain metastasis. [33]
High rates of neurologic death have been reported after stereotactic radiosurgery (SRS) failure, particularly for recurrences deep in the brain and surgically inaccessible. Laser interstitial thermal therapy (LITT) is an emerging option in this setting, but its ability to prevent neurologic death is unknown. In a single-institution retrospective case series of patients with BM who underwent LITT for in-field recurrence after SRS, investigators collected clinical and demographic data through chart review. The primary endpoint was cause of death. Analysis revealed that 49 patients had died; cause of death was identified for 44. Death was neurologic in 20 patients and non-neurologic in 24. The 24-month cumulative incidence of neurologic and non-neurologic death was 35.1% and 38.6%, respectively. Causes of neurologic death included local recurrence (n = 7), recovery failure (n = 7), distant progression (n = 5), and other (n = 1). In this patient population, LITT effectively stabilized neurologic disease in up to 2 of 3 patients. Study authors concluded that for in-field recurrence after SRS, LITT may represent a reasonable treatment strategy for select patients. However, additional research is needed to determine the extent to which LITT can prevent neurologic death after BM recurrence. [2]
-
Sagittal T1-weighted precontrast and postcontrast MRI. A surgically proven, solitary right parietal metastasis is seen with a single, 1-cm, enhancing right parietal nodule and extensive surrounding edema typical of a metastasis.
-
Contrast-enhanced CT shows multiple enhancing metastatic nodules at the gray-white junction in a patient with known colon cancer.
-
Precontrast- and postcontrast-enhanced CT shows multiple ring-enhancing lesions (thick, peripheral, ringlike) in the left hemisphere with prominent surrounding edema and mass effect in a patient with known lung cancer.
-
Coronal contrast-enhanced T1-weighted MRI. Multiple solid and cystic supratentorial and infratentorial mass lesions are seen in a patient with known breast cancer. Note the cystic, ring-enhancing, and solid patterns.
-
Axial contrast-enhanced T1-weighted MRI of a patient with known lung cancer. Multiple, small ring-enhancing lesions are seen in both hemispheres.
-
Sagittal contrast-enhanced MRI of a patient with known prostate cancer. The solitary enhancing right parietal extra-axial lesion is a surgically proven dural metastasis.
-
Coronal contrast-enhanced MRI of a patient with known melanoma. Note linear enhancement in the sulci and brain surface of the right frontal and temporal lobes suggestive of leptomeningeal carcinomatosis. The cancer was proven by cerebrospinal fluid cytology.
-
Precontrast- and postcontrast-enhanced CT of a patient with breast cancer. Linear enhancement in the anterior falx and medial sulci of both frontal lobes suggests leptomeningeal and dural carcinomatosis.
-
Noncontrast CT of a patient with lung cancer. Multiple high-density lesions are seen in both cerebral hemispheres, suggestive of hemorrhagic metastases.
-
Contrast-enhanced CT and bone CT of a patient with breast cancer. Multiple irregular and aggressive lytic lesions are seen in the calvarium with dural metastases.
-
Precontrast- and postcontrast-enhanced CT scan of a patient with mucin-secreting adenocarcinoma of the stomach. Multiple calcified nodules are seen in the surgically proven metastasis.
-
T2-weighted and postcontrast T1-weighted MRI of a patient with uterine cancer. Well-defined, nonenhancing, round lesion with fluid signal intensity is seen in the right temporal lobe, with prominent surrounding edema. This is a surgically proven cystic metastasis.










