Practice Essentials
A solitary pulmonary nodule (SPN) is defined as a single, discrete pulmonary opacity that is surrounded by normal lung tissue and is not associated with adenopathy or atelectasis. Radiographically, a nodule is defined as a lesion smaller than 3 cm. Anything larger than 3 cm is termed a mass. [1, 2, 3, 4] The finding of an SPN on a chest radiograph is a diagnostic dilemma faced by many clinicians. The differential diagnosis may be broad, but implications rest on whether the lesion is benign or malignant. The only findings sufficient to preclude further evaluation are a benign pattern of calcification or stability of nodule size for over 2 years for solid pulmonary nodules. The likelihood of malignancy increases with nodule size, which may influence management strategy. Other nodule features such as shape, edge characteristics, cavitation, and location have not yet been found to be accurate clues for distinguishing benign from malignant nodules. As a result, the majority of nodules are indeterminate. [1]
The probability of malignancy can be assessed clinically or by quantitative predictive models as falling into 1 of 3 risk categories: very low probability (< 5%), low/moderate probability (5-65%), or high probability (>65%). Quantitative predictive models combine clinical and radiologic features to estimate malignancy potential. The most commonly used model, from the Mayo Clinic, estimates the probability of malignancy using 6 independent predictors: smoking history, older age, history of extrathoracic cancer more than 5 years before nodule detection, nodule diameter, spiculation presence, and upper lobe location. [5]
Imaging modalities
Most solitary pulmonary nodules are incidental findings on imaging studies of the chest, abdomen, and upper extremities. Occasionally, nodules as small as 5-6 mm can be visualized on chest radiography. The imaging tools that are used to evaluate solitary pulmonary nodules include chest CT and functional imaging (usually fluorodeoxyglucose–positron emission tomography, or FDG-PET). Chest CT, preferably with thin sections, should be obtained in all patients with unclearly characterized solitary pulmonary nodules visible on chest radiography.
The advantages of CT scanning over radiography include better resolution of nodules and detection of nodules as small as 3-4 mm. CT scan images also help to better characterize the morphologic features of various lesions. Multiple nodules and regions that are difficult to assess on chest radiographs are better visualized on CT scan images. [6] Contrast enhancement is not typically required when imaging a solitary nodule. [1]
CT is the imaging modality of choice for reevaluation of pulmonary nodules visible on chest radiography and for continued surveillance of nodules for change in size. Radiologic features such as size, border, density, calcification, and growth rate are used to predict malignancy.
Chest radiographs demonstrate poorer resolution than chest CT scans in determining degree of calcification or size. Visualization of some nodules may be difficult because of superimposed structures.
FDG-PET scans have several limitations because false-positive findings can occur in other infectious or inflammatory conditions that yield metabolically active pulmonary nodules. Moreover, tumors that have lower metabolic rates (such as carcinoid, lepidic-predominant adenocarcinomas and mucinous adenocarcinomas) may be difficult to distinguish from background activity and hence yield false-negative results. FDG-PET scans have lower sensitivity for nodules smaller than 20 mm in diameter and may miss lesions smaller than 10 mm.
When staging lung cancer, MRI provides better imaging for pleural, diaphragm, and chest wall disease than does CT scanning. MRI is comparable to CT in assessing mediastinal involvement and is less useful in assessing the lung parenchyma (especially in assessing pulmonary nodules) because of poorer spatial resolution. Since MRI costs more and is less available, MRI use is reserved for tumors that are difficult to assess on CT (eg, Pancoast tumors).
(The radiologic features of SPNs are demonstrated in the images below.)
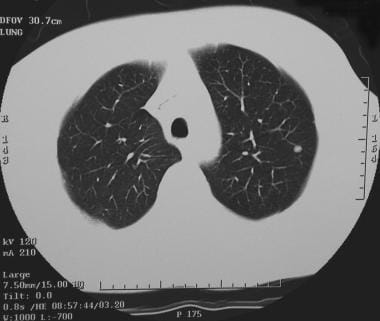
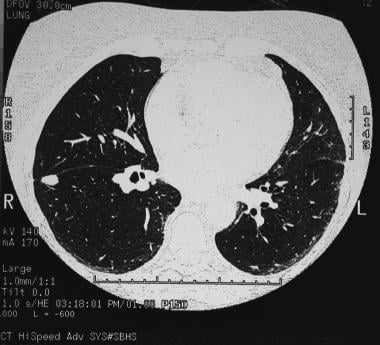 A solitary pulmonary nodule in the right lower lobe adjacent to the fissure in the periphery. Biopsy confirmed the diagnosis of a coccidioidoma.
A solitary pulmonary nodule in the right lower lobe adjacent to the fissure in the periphery. Biopsy confirmed the diagnosis of a coccidioidoma.
Lung cancer screening and guidelines
Annual low-dose computed tomography (LDCT) screening for lung cancer in high-risk current and former smokers has become a standard of care in the United States, in large part due to the results of the National Lung Screening Trial (NLST). The NLST included 53,452 current or former smokers aged 55 to 74 years with at least a 30 pack-year history of cigarette use. Former smokers had to have quit within the past 15 years. The results showed a 16% reduction in lung cancer-specific mortality (per 100,000 person years). [7] LDCT screening for lung cancer has led to the detection of more small nodules requiring evaluation.
Concerns associated with LDCT screening include false-negative and false-positive results, incidental findings, overdiagnosis, and cumulative radiation exposure. False-positive LDCT results occur in a substantial proportion of screened persons. While further imaging does resolve most false-positive results, some patients may require invasive procedures. Kinsinger and colleagues reported that of 2106 individuals who were screened according to US Preventive Services Task Force (USPSTF) guidelines, 1257 (59.7%) had a positive test result, including 1184 (56.2%) with one or more nodules that needed to be tracked. [8, 9]
The USPSTF recommends annual screening with low-does CT in adults aged 50-80 years (previously 55-80 yr) who have a 20 pack-year smoking history (reduced from 30 pack-years) and currently smoke or who have quit smoking within the past 15 years. Additionally, they recommend that screening be stopped once a person has not smoked for 15 years or has a health problem that limits life expectancy or the ability to have lung surgery. [10] The American College of Radiology guidelines are in agreement with the USPSTF recommendations. [11]
The National Comprehensive Cancer Network (NCCN) also recommends LDCT screening for high-risk patients aged 50 years or older with a 20 pack-year or greater smoking history. In addition, the NCCN recommends considering other potential risk factors for lung cancer, including occupational exposure, radon exposure, cancer history, family history, lung disease history, and second-hand smoke exposure. [12]
The American College of Chest Physicians (ACCP) guidelines for lung cancer include the following [13, 14] :
-
LDCT should be offered to asymptomatic smokers and former smokers aged 55-77 yr who have smoked 30 pack-years or more and either continue to smoke or have quit within the past 15 yr .
-
LDCT screening should not be routinely performed in asymptomatic smokers and former smokers who do not meet the smoking and age criteria for screening but are deemed to be at high risk for lung cancer based on clinical risk prediction calculators, .
-
Screening programs should develop strategies to determine whether patients have symptoms that suggest the presence of lung cancer, so that symptomatic patients do not enter screening programs but instead receive appropriate diagnostic testing, regardless of whether the symptomatic patient meets screening eligibility criteria.
-
Screening programs should define what constitutes a positive test on LDCT based on the size of a detected solid or part-solid lung nodule, with a threshold for a positive test that is either 4 mm, 5 mm, or 6 mm in diameter. A positive test is defined as a test that leads to a recommendation for any additional testing other than to return for the annual screening exam.
-
Screening programs should develop a comprehensive approach to lung nodule management, including multidisciplinary expertise and algorithms for the management of small solid nodules, larger solid nodules, and subsolid nodules. Strategies should be developed to minimize overtreatment of potentially indolent lung cancers .
In the revised Fleischner Society guidelines, the minimum threshold size for routine follow-up has been increased, and recommended follow-up intervals are now given as a range rather than as a precise time period to give radiologists, clinicians, and patients greater discretion to accommodate individual risk factors and preferences. [15]
The British Thoracic Society (BTS) guidelines include 4 management algorithms and 2 malignancy prediction calculators to help measure the risk of malignancy. To help reduce the number of follow-up CT scans performed, the BTS recommendations include a higher nodule size threshold for follow-up and a reduction of the follow-up period to 1 year for solid pulmonary nodules. Volumetry is recommended as the preferred measurement method. [16]
In an effort to standardize the manner in which low-dose lung cancer screening CT scans are reported, the American College of Radiology (ACR) introduced Lung-RADS [17] , as follows:
-
Categor 0 is considered incomplete and requires additional screening or comparison to prior chest CT exam.
-
Category 1 corresponds to no nodules or definitely benign nodules; continue annual low-dose CT; risk of malignancy < 1%.
-
Category 2 corresponds to benign appearance or behavior; continue annual low-dose CT; risk of malignancy < 1%.
-
Category 3 corresponds to nodules that are probably benign, with nodules that have a low likelihood of becoming clinically active cancer; perform LDCT every 6 months; risk of malignancy 1-2%.
-
Category 4a is considered suspicious, with additional testing recommended; 3 month LDCT; PET/CT may be used when there is a ≥8 mm solid component; risk of malignancy 5-15%.
-
Category 4b is considered very suspicious, with additional testing or tissue sampling recommended; chest CT with or without contrast; PET/CT and/or tissue sampling, depending on the probability of malignancy and comorbidities. PET/CT may be used when there is a ≥8 mm solid component. For new large nodules that develop on an annual repeat screening CT, a 1-month LDCT may be recommended to address potentially infectious or inflammatory conditions; risk of malignancy >15%.
-
Category 4x is same as 4b but corresponds to category 3 or 4 nodules with additional features or imaging findings that increase the suspicion of malignancy.
Radiography
Often, solitary pulmonary nodules (SPNs) are discovered first as incidental findings on chest radiographs. The first step is to determine whether the nodule is pulmonary or extrapulmonary. A lateral radiograph, fluoroscopy, or CT scanning of the chest often helps determine the location of the nodule. [6, 18]
Usually, nodules are identifiable by the time they are 8-10 mm on chest radiographs. Occasionally, SPNs can be visualized at 5 mm in diameter. Chest radiographs can provide information regarding nodule size, growth rate, margin characteristics, and calcification pattern, which can aid in the assessment of benign versus malignant lesions.
Some SPN mimics include nipple shadows, soft tissue tumors, bone shadows, pleural plaques, pseudotumors, and round atelectases.
(The different types of solitary pulmonary nodules are seen in the radiographs below.)
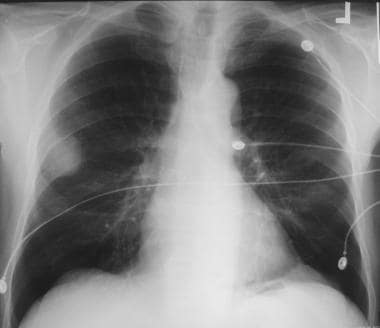 Solitary pulmonary nodule. Large well-circumscribed mass in the periphery of the right upper lobe later was determined to be a neurilemoma.
Solitary pulmonary nodule. Large well-circumscribed mass in the periphery of the right upper lobe later was determined to be a neurilemoma.
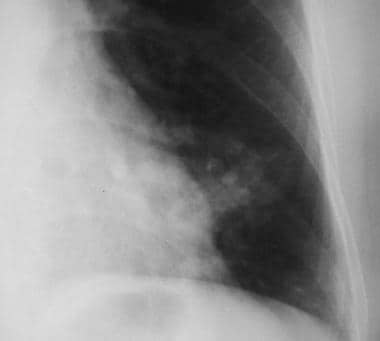 Solitary pulmonary nodule. Close-up view of a pulmonary arteriovenous malformation. Note the feeding vessels.
Solitary pulmonary nodule. Close-up view of a pulmonary arteriovenous malformation. Note the feeding vessels.
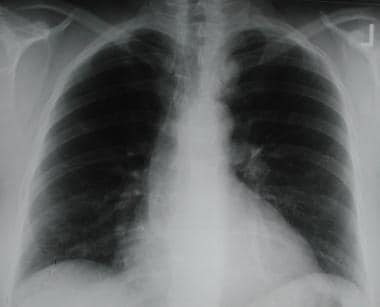 Solitary pulmonary nodule. Posteroanterior chest radiograph shows a mass lesion abutting the left upper mediastinum. Features are noted that suggest this is a mediastinal rather than a parenchymal lesion (obtuse margins, continuation of opacity).
Solitary pulmonary nodule. Posteroanterior chest radiograph shows a mass lesion abutting the left upper mediastinum. Features are noted that suggest this is a mediastinal rather than a parenchymal lesion (obtuse margins, continuation of opacity).
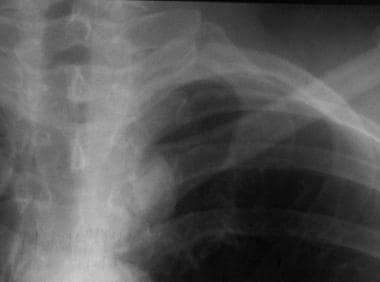 Solitary pulmonary nodule. Close-up view highlights features that suggest this is a mediastinal tumor.
Solitary pulmonary nodule. Close-up view highlights features that suggest this is a mediastinal tumor.
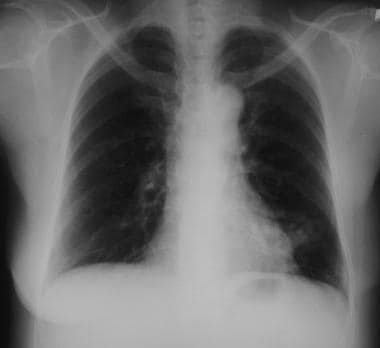
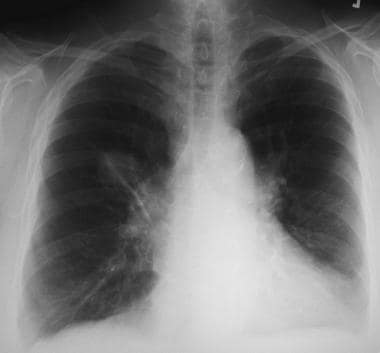 Solitary pulmonary nodule. Right upper lobe nodule in a lifelong smoker. Percutaneous needle biopsy confirmed a diagnosis of adenocarcinoma.
Solitary pulmonary nodule. Right upper lobe nodule in a lifelong smoker. Percutaneous needle biopsy confirmed a diagnosis of adenocarcinoma.
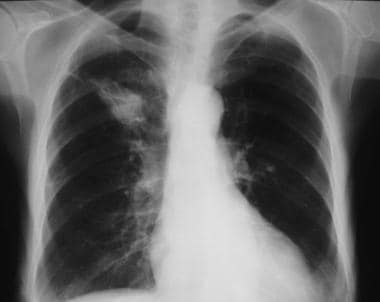 Right upper lobe mass. Features of the lesion are not in keeping with the definition of solitary pulmonary nodule and the mass is likely to be a malignant lesion. In this patient, the lesion was squamous cell carcinoma.
Right upper lobe mass. Features of the lesion are not in keeping with the definition of solitary pulmonary nodule and the mass is likely to be a malignant lesion. In this patient, the lesion was squamous cell carcinoma.
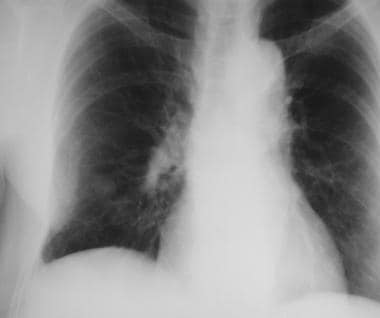 A solitary pulmonary nodule along the rib can be a callus formation secondary to rib fracture. Oblique views may be helpful.
A solitary pulmonary nodule along the rib can be a callus formation secondary to rib fracture. Oblique views may be helpful.
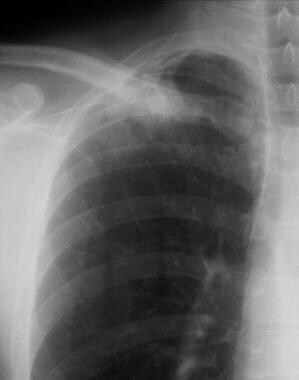
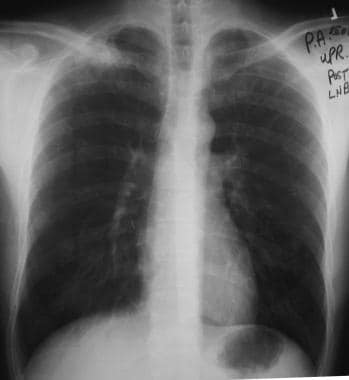 Solitary pulmonary nodule. Special attention must be paid to the apices so as not to miss the lesion in these areas. Apical lordotic views can demonstrate the lesions well.
Solitary pulmonary nodule. Special attention must be paid to the apices so as not to miss the lesion in these areas. Apical lordotic views can demonstrate the lesions well.
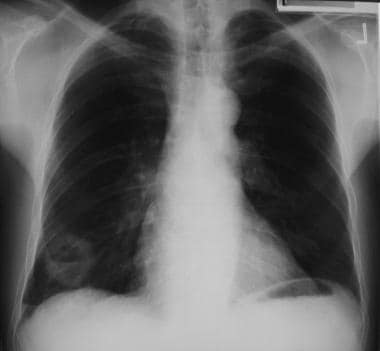
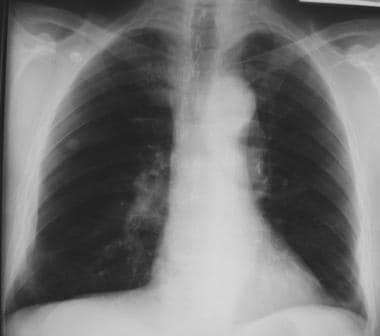 Solitary pulmonary nodule. Findings show a right-sided pulmonary nodule. The differential is long; however, CT scan may be helpful in narrowing the differential diagnosis.
Solitary pulmonary nodule. Findings show a right-sided pulmonary nodule. The differential is long; however, CT scan may be helpful in narrowing the differential diagnosis.
Nodule size
Nodules greater than 3 cm in diameter are more likely to be malignant, while those less than 2 cm are more likely to be benign. Note that size alone is of limited value. In individual patients, small nodules can be malignant and larger nodules can be benign.
Growth rate
Comparison of previous chest radiographs of the patient allows assessment of the growth rate. The growth rate refers to the doubling time of a nodule (ie, the time necessary for the nodule's volume to double). On chest radiographs, a nodule appears as a 2-dimensional representation of a 3-dimensional structure. The volume of a sphere equals 4/3 pr3; therefore, a 26% increase in diameter on a chest radiograph represents one doubling in volume. For example, an increase from 1.0 cm to 1.3 cm equals one doubling, and a 1 cm to 2 cm increase relates to an 8-fold increase in volume.
Bronchogenic carcinomas usually have a doubling time of 20-400 days. Doubling times shorter than 20-30 days are seen in infections, infarction, lymphoma, or fast-growing metastases.
Doubling times greater than 400 days are typically indicative of benign nodules, although on occasion, a low-grade carcinoid tumor may have a doubling time greater than 400 days.
Absence of a change in size of a nodule over 2 years is highly suggestive of a benign lesion.
Determination of the size of small nodules is not without error. On chest radiographs, a 3-mm enlargement may be difficult to appreciate. The use of digitally enhanced films may allow for more accurate measurements of size.
Margin characteristics
Benign lesions tend to have well-circumscribed, smooth borders. Malignant nodules typically have irregular, lobulated, or spiculated (corona radiata) borders. Of the margin descriptions, the spiculated border is the most sensitive in predicting malignancy; however, it is not unusual for a malignant lesion to have a smooth contour.
Calcification
Calcification within a nodule is more likely to be seen in a benign nodule; however, approximately 10% of malignant nodules demonstrate calcification. In benign lesions, 5 patterns of calcification are commonly seen: diffuse, central, laminar, concentric, and popcorn (chondroid). The popcorn pattern typically is described in hamartomas (an example of which appears below). A stippled or eccentric pattern is most commonly seen in malignant lesions. CT scanning allows a more accurate detection and assessment of the calcification pattern than plain films do.
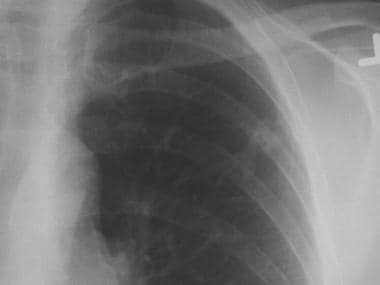 Chest radiograph of a left upper lobe solitary pulmonary nodule. Biopsy demonstrated this to be hamartoma.
Chest radiograph of a left upper lobe solitary pulmonary nodule. Biopsy demonstrated this to be hamartoma.
Computed Tomography
CT scanning of the chest allows better assessment of nodules than does plain radiography. [19, 20] Nodules as small as 3-4 mm are detectable on CT scans, and morphologic features of a specific diagnosis are better visualized (eg, rounded atelectasis, arteriovenous malformations).
Areas that are difficult to assess on plain radiographs are also better visualized on CT scans, including the lung apices, perihilar regions, and costophrenic angles.
In addition, multiple nodules can be detected on CT scans, malignancy can be staged using the modality, and CT scanning can help guide needle biopsy.
(The characteristics and types of solitary pulmonary nodules, as seen on CT scans, are demonstrated in the images below.)
 A solitary pulmonary nodule in the right lower lobe adjacent to the fissure in the periphery. Biopsy confirmed the diagnosis of a coccidioidoma.
A solitary pulmonary nodule in the right lower lobe adjacent to the fissure in the periphery. Biopsy confirmed the diagnosis of a coccidioidoma.
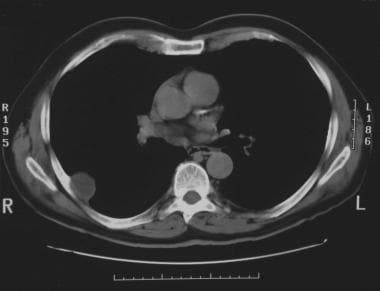 Solitary pulmonary nodule. CT scan of the chest showing neurilemoma. Note how the neurilemoma arises adjacent to the rib.
Solitary pulmonary nodule. CT scan of the chest showing neurilemoma. Note how the neurilemoma arises adjacent to the rib.
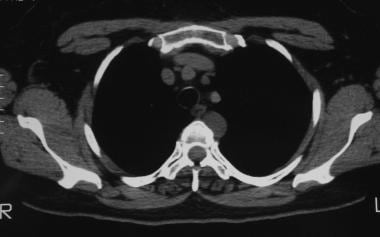 Solitary pulmonary nodule. CT scan that shows a lesion to be posterior mediastinal. The differential diagnosis includes neurogenic tumors, mediastinal cysts, malignancy, and infectious lesions.
Solitary pulmonary nodule. CT scan that shows a lesion to be posterior mediastinal. The differential diagnosis includes neurogenic tumors, mediastinal cysts, malignancy, and infectious lesions.
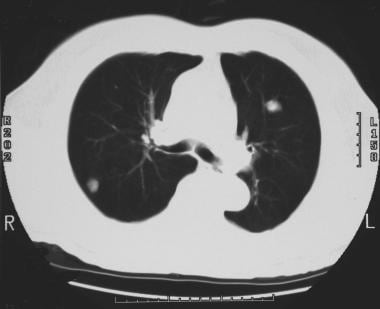 Solitary pulmonary nodule. CT scan of the thorax. Multiple pulmonary nodules were identified. The differential diagnosis includes metastasis and infectious granulomas.
Solitary pulmonary nodule. CT scan of the thorax. Multiple pulmonary nodules were identified. The differential diagnosis includes metastasis and infectious granulomas.
CT densitometry
CT densitometry measures the attenuation coefficients of a particular lesion to determine its density. The results are expressed in Hounsfield units (HU). Some examples of attenuation coefficients are as follows:
-
Air: -1000 HU
-
Fat: -50 to -100 HU
-
Water: 0 HU
-
Blood: 40-60 HU
-
Noncalcified nodule: 60-160 HU
-
Calcified nodule: Greater than 200 HU
-
Bone: 1000 HU
CT densitometry allows for the detection of occult calcification that may not be appreciated visually, even on high-resolution thin-section CT of the chest. The difficulties with this technique have been in determining the appropriate level of the attenuation coefficients used to classify a lesion with a high probability of being benign.
One study looking at 91 nodules known to be malignant or benign proposed a cutoff of greater than 164 HU for benign lesions. In another study, of 85 nodules classified as benign (using 185 HU as a cutoff), 9% were found to be malignant at biopsy. Densitometry in this setting may provide useful information if used in context with other clinical and radiologic features, but overall, its use has fallen out of favor.
Densitometry also allows detection of fat within a nodule, which is a common feature of benign nodules, especially in hamartomas.
Contrast enhancement
Malignant nodules tend to have greater vascularity than do benign nodules. Assessment of enhancement involves repeated measurement of attenuation of a nodule over a 5-minute period. Nodular enhancement of less than 15 HU suggests that a lesion is benign, and enhancement of greater than 20 HU is more likely associated with malignancy (sensitivity 98%, specificity 73%).
Feeding vessel sign
This sign may be seen in hematogenous or vascular causes of pulmonary nodules, such as metastatic deposits or septic emboli.
Cavity wall thickness
Cavitation can be seen in malignant and benign nodules. While a thin-walled cavity is highly suggestive of a benign lesion (1 mm or less), a thick-walled cavity is usually indeterminate and is present in benign and malignant lesions.
Nuclear Imaging
PET and SPECT scanning
Malignant cells have a higher metabolic rate than do normal cells; therefore, glucose uptake is higher. Thoracic PET imaging uses the isotope fluorine-18 bound to a glucose analog to make fluorine-18-fluorodeoxyglucose (FDG). Increased FDG uptake is seen in most malignant tumors and is the basis of the PET study used to differentiate malignant from benign nodules. [21, 22] FDG uptake can be quantified using the standardized uptake ratio (SUR) to normalize measurements for a patient's weight and injected dose of radioisotope. This allows comparison of uptake between different lesions and patients. According to the guidelines of the American College of Chest Physicians (ACCP), a standardized uptake value greater than 2.5 is used to identify nodules that have a high probability of malignancy. [14]
FDG-PET is most cost-effective when the clinical pretest probability of malignancy and the results of the CT are discordant (eg, low pretest probability with chest CT characteristics that are clearly not benign). The ACCP guidelines recommend FDG-PET in persons with solid indeterminate nodules 8 mm or greater in diameter, along with a low to intermediate pretest probability of malignancy. [14] An additional advantage of FDG-PET imaging is better detection of mediastinal metastases, improving the staging of lung cancers.
High serum glucose concentrations compete in cells with FDG; therefore, uptake of the radioisotope is reduced. In a meta-analysis, the mean sensitivity and specificity for detecting malignancy in focal pulmonary lesions of any size were 96% and 73.5%, respectively. In SPNs, the mean sensitivity and specificity were 93.9% and 85.8%, respectively. [21]
In FDG-PET scanning, false-positive findings can occur in other metabolically active conditions that produce pulmonary nodules, such as infectious granulomas and inflammatory lesions.
The resolution of PET scanners is 7-8 mm; therefore, they may miss tumors smaller than 10 mm.
In FDG-PET scanning, tumors with low metabolic rates, such as carcinoid tumors and bronchioalveolar cell carcinomas, may not be distinguishable from background uptake.
SPECT scanners have the advantage of being more readily available than PET scanners. Depreotide is a somatostatin analog labeled with technetium-99m (99mTc), which has been shown to bind to somatostatin receptors expressed on non-small-cell carcinomas. In a study of a small series of patients, depreotide uptake demonstrated a sensitivity and specificity of 93% and 88%, respectively, for malignancy. [23]
-
Solitary pulmonary nodule. Cavitating nodule secondary to an abscess.
-
A solitary pulmonary nodule in the right lower lobe adjacent to the fissure in the periphery. Biopsy confirmed the diagnosis of a coccidioidoma.
-
Chest radiograph of a left upper lobe solitary pulmonary nodule. Biopsy demonstrated this to be hamartoma.
-
CT scan of a small solitary pulmonary nodule in the left upper lobe.
-
Solitary pulmonary nodule. CT scan of the chest showing neurilemoma. Note how the neurilemoma arises adjacent to the rib.
-
Solitary pulmonary nodule. Neurilemoma - Lung window view.
-
Solitary pulmonary nodule. Large well-circumscribed mass in the periphery of the right upper lobe later was determined to be a neurilemoma.
-
Solitary pulmonary nodule. Pulmonary arteriovenous malformation in left lower lobe.
-
Solitary pulmonary nodule. Close-up view of a pulmonary arteriovenous malformation. Note the feeding vessels.
-
Solitary pulmonary nodule. Posteroanterior chest radiograph shows a mass lesion abutting the left upper mediastinum. Features are noted that suggest this is a mediastinal rather than a parenchymal lesion (obtuse margins, continuation of opacity).
-
Solitary pulmonary nodule. Close-up view highlights features that suggest this is a mediastinal tumor.
-
Solitary pulmonary nodule. CT scan that shows a lesion to be posterior mediastinal. The differential diagnosis includes neurogenic tumors, mediastinal cysts, malignancy, and infectious lesions.
-
Solitary pulmonary nodule. Right upper lobe nodule in a lifelong smoker. Percutaneous needle biopsy confirmed a diagnosis of adenocarcinoma.
-
Right upper lobe mass. Features of the lesion are not in keeping with the definition of solitary pulmonary nodule and the mass is likely to be a malignant lesion. In this patient, the lesion was squamous cell carcinoma.
-
A solitary pulmonary nodule along the rib can be a callus formation secondary to rib fracture. Oblique views may be helpful.
-
Solitary pulmonary nodule. Special attention must be paid to the apices so as not to miss the lesion in these areas. Apical lordotic views can demonstrate the lesions well.
-
Solitary pulmonary nodule. Close-up chest radiograph (same patient as in the previous image).
-
Solitary pulmonary nodule. Findings show a right-sided pulmonary nodule. The differential is long; however, CT scan may be helpful in narrowing the differential diagnosis.
-
Solitary pulmonary nodule. CT scan of the thorax. Multiple pulmonary nodules were identified. The differential diagnosis includes metastasis and infectious granulomas.