Practice Essentials
Wilms tumor, or nephroblastoma, is the most common solid renal mass and abdominal malignancy of childhood, with a prevalence of 1 case per 10,000 population. Conversely, it is very rare in adults, with an incidence rate of less than 0.2 per million per year. The tumor (see the images below) occurs in both hereditary and sporadic forms, and approximately 6% are bilateral. Most are unicentric and arise from the kidney. Extrarenal Wilms tumors (EWTs) are rare and most commonly found in the retroperitoneal space (44.4%) and the uterus (14.8%). The results of treatment of Wilms tumor have improved, reaching 4-year overall survival in the favourable histology group of nearly 90%. [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]
Most children with Wilms tumor will present with signs of a renal condition, including abdominal swelling or a suspicious mass. The abdominal mass may be discovered by a caretaker during routine activities such as bathing or by a pediatrician during examination. In most cases, there will be a solitary tumor in one kidney, but 5-13% of children have bilateral tumors and 10% have multifocal tumors in a single kidney. [9]
Imaging is particularly important in surgical planning. MRI diffusion studies have been shown to help differentiate Wilms tumor from neuroblastoma, with the apparent diffusion coefficient (ADC) being substantially higher for Wilms tumor. [8] Ultrasonography of the abdomen is often performed before a more definitive CT scan with contrast or MRI with contrast of the abdomen. A CT scan of the abdomen confirms the renal origin of the tumor and determines the presence of bilateral tumors. About 5% of renal masses that are initially thought to be Wilms tumor are subsequently diagnosed as another condition. Approximately 15-20% of patients will present with metastases, with common sites of metastases being the lungs (85%) and liver (10%). CT scanning is the most sensitive imaging modality for detecting metastatic lung nodules. If chest CT is performed initially, chest radiography is not necessary. [11, 12, 13]
Imaging modalities
Although modern imaging techniques such as color Doppler sonography, helical or multidetector-row CT, and MRI have substantially improved the potential to image Wilms tumors, definitive diagnosis is still based on histology. Children presenting with abdominal masses initially undergo ultrasonography scanning, usually in combination with chest radiography. Initial diagnosis of a Wilms tumor is generally based on ultrasonography supplemented with Doppler ultrasound because inferior vena cava (IVC) tumoral thrombi are occasionally missed on CT; missing these thrombi can lead to a fatal outcome at surgery. If a Wilms tumor is suspected or if the primary tumor is histologically confirmed, it should be staged by using CT or MRI. The tumors may be large, and their size may make it difficult to identify its renal origin on sonograms. Therefore, CT and MRI may be useful for distinguishing between renal tumors and adrenal tumors. Radiologists prefer chest CT over chest radiography to stage the spread of disease to the thorax. [14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27]
In a study of ultrasound and laboratory findings in Wilms tumor survivors with a solitary kidney, signs of kidney damage were seen in 22 of 53 patients (41.5%) on ultrasonography. The most frequently detected abnormalities were hyperechoic rings around renal pyramids (28.3% of patients). Hypertrophy of the solitary kidney occurred in 71,7% of cases. [28]
Conventional radiography is inexpensive and noninvasive; however, it has low sensitivity and specificity. A chest radiograph may miss lung metastases. Regarding CT and MRI, sedation or general anesthesia may be required. MRI is expensive and has certain contraindications; for example, claustrophobia may be a problem. Neither CT nor MRI is tissue specific, and tissue diagnosis may be required. Bone scintigraphic studies are highly sensitive but lack specificity.
Performing chest CT to stage a Wilms tumor can be justified only if the following conditions are met [29] :
-
If the information obtained is reliable
-
If the CT findings are correlated with the patient's prognosis
-
If the CT results alter treatment from what would have been recommended in their absence
-
If the information obtained with CT favorably influences the therapeutic outcome
The main objectives of staging Wilms tumors are the following:
-
To identify the origin of the tumor
-
To assess the extent of the tumor
-
To assess involvement of the renal vascular pedicle
-
To detect regional lymph node metastases
-
To detect bilateral Wilms tumors
-
To detect distant metastases
CT or MRI fulfills these objectives well. Although CT of the chest may be included in the primary staging procedure, most investigators from international studies of Wilms tumors have relied on chest radiographs to detect lung metastases.
Angiography is uncommonly performed, but it may be useful in the preoperative assessment of tumors in patients with a solitary kidney or bilateral Wilms tumors. Likewise, inferior venacavography is now seldom performed, as magnetic resonance angiography (MRA) and Doppler imaging studies may provide the same information noninvasively.
Radionuclide studies may be indicated for assessing the volume of functioning renal tissues by using technetium-99m dimercaptosuccinic acid (DMSA). The findings may provide guidance as to what tissues may be preserved when nephron-sparing surgery of bilateral tumors is being contemplated. Isotope renography is a sensitive technique for assessing renal function.
Positron emission tomography (PET) may be important in the future. Bone scintigraphy is useful for evaluating only clear cell sarcoma, which tends to metastasize to bone.
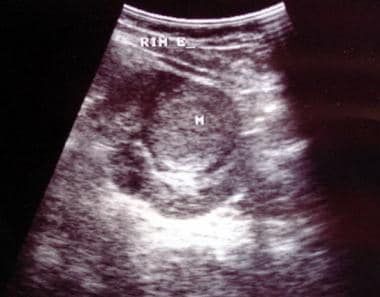
 This 6-year-old male child with hematuria was referred for a renal ultrasound scan. The scan shows a 6 x 8 cm solid mass at the lower pole of the right kidney displacing part of the collecting system in a cephalad direction. The mass is of uniform echogenicity with a vague small central hypoechoic area suggestive of tumor necrosis.
This 6-year-old male child with hematuria was referred for a renal ultrasound scan. The scan shows a 6 x 8 cm solid mass at the lower pole of the right kidney displacing part of the collecting system in a cephalad direction. The mass is of uniform echogenicity with a vague small central hypoechoic area suggestive of tumor necrosis.
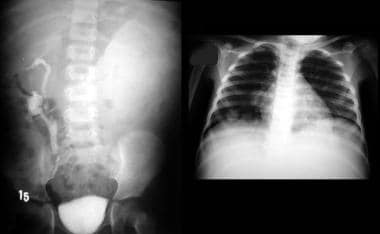 An IVU shows a nonfunctioning left kidney with a suggestion of ill-defined mass in the left loin due to a biopsy-proven Wilms tumor. Note the functioning right duplex renal collecting system. The chest radiograph in the same child shows a lung metastatic deposit (arrow). Images courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
An IVU shows a nonfunctioning left kidney with a suggestion of ill-defined mass in the left loin due to a biopsy-proven Wilms tumor. Note the functioning right duplex renal collecting system. The chest radiograph in the same child shows a lung metastatic deposit (arrow). Images courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
 Axial US image shows a solid 4.5-cm solid mass anterior cortex, lower pole of the left kidney. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
Axial US image shows a solid 4.5-cm solid mass anterior cortex, lower pole of the left kidney. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
Surgical resection is mandatory for treatment and staging, with all patients receiving chemotherapy. Radiation therapy is reserved for managing residual abdominal tumors or hematogenous metastatic disease. The survival rate has been a remarkable 90% with a multidisciplinary approach to this tumor. Radiologic diagnosis, staging, and follow-up are crucial for therapeutic success. About 15% of treated patients have relapses of Wilms tumor, with most occurring within 2 years after nephrectomy and only occasionally after 5 years. [30]
Surgical staging
Wilms tumors are usually staged by using the method suggested in the National Wilms Tumor Studies (NWTS), in which the tumors are classified for surgery into 5 stages, as described below. [31, 32, 33]
Stage I
The stage I tumor is confined to the kidney and is completely excised. The renal capsule is intact. No evidence of tumor at or beyond the resection margins is noted. The tumor is not ruptured, or it was sampled during biopsy (excluding fine-needle aspiration biopsy) before its surgical removal. The vessels of the renal sinus are free from disease.
Stage II
The stage II tumor extends beyond the kidney and is completely excised. The renal capsule or renal sinus may be invaded. The renal vascular pedicle may contain tumor. The tumor was previously sampled during biopsy (except for fine-needle aspiration biopsy), or the tumor spilled before or during surgery, but the spillage was confined to the renal fossa and does not involve the peritoneal surface. No evidence of tumor at or beyond the resection margins is noted.
Stage III
In stage III tumor, residual nonhematogenous tumor is present after surgery, and the tumor is confined to the abdomen. Any of the following situations may occur:
-
Lymph node findings in the abdomen or pelvis are positive.
-
The tumor penetrates the peritoneal surface, or implants are found on the peritoneal surface.
-
Tumor cells are found at the margin of surgical resection on microscopic examination.
-
Tumor spills beyond the flank before or during surgery.
Stage IV
In stage IV, there are hematogenous metastases—involving, for example, the lung, liver, bone, and brain—or lymph node metastases outside the abdominopelvic region.
Stage V
In stage V tumor, bilateral renal tumoral involvement is present.
Differential diagnosis
Renal blastema and/or nephroblastomatosis
Renal blastema and nephroblastomatosis are interrelated conditions closely related to Wilms tumors. The persistence of primitive renal blastema beyond infancy (4 mo) is abnormal except in small microscopic rests. Large amounts of primitive blastema remaining in sheets in the cortex or in discrete nodules are termed nephroblastomatosis.
Ultrasonographic detection is possible, but sonography lacks the sensitivity of CT and MRI. On sonograms, the affected kidney may be enlarged and lobulated with multiple hypoechoic areas. Corticomedullary differentiation may be lost.
After such findings are discovered, 3 monthly ultrasound examinations should be performed to detect their progression to a Wilms tumor. Rapid growth of any of the hypoechoic rests suggests progression. Antenatal detection is possible when sonograms reveal bilateral nephromegaly with normal renal echogenicity. However, foci of calcification may be associated with polyhydramnios, and they can occur as a part of a familial condition.
Mesoblastic nephroma
Mesoblastic nephroma is the most common congenital renal neoplasm. It is a solitary hamartoma, and it is usually benign and unilateral. Sonograms show mesoblastic nephroma as a complex mass that may contain cystic areas. A Wilms tumor may also appear as a multiloculated mass. Antenatal diagnosis is possible. On sonograms, mesoblastic nephroma is seen as a large, solitary, predominantly solid, coarse, and echogenic renal mass that may contain cystic areas. It may be associated with polyhydramnios.
Clear cell sarcoma
Clear cell sarcoma is a distinct histologic entity that accounts for 4% of the renal tumors observed in childhood. These tumors usually cannot be differentiated from Wilms tumors on images. Clear cell sarcoma is not associated with any other somatic abnormality. Overall, the prognosis is poor compared to that of patients with Wilms tumors. Since bone metastases may occur with this disease, bone scintigraphy or FDG-PET is recommended.
Rhabdoid tumor of the kidney
The term rhabdoid is derived from the microscopic appearance of the tumor cells that resemble muscle cells. Rhabdoid tumors make up around 2% of renal neoplasms and are more common in infancy than in any other period. An association with brain tumors, especially medulloblastoma, has been described. The brain tumors may precede or appear several years after the detection of this tumor. Brain MRI is needed as part of the workup of rhabdoid tumors.
Like other neonatal masses, rhabdoid tumor of the kidney can be diagnosed in utero. In neonates, detection may follow their presentation with an abdominal mass, hypertension, or hypercalcemia. The age at presentation overlaps with that noted for congenital mesoblastic nephroma. The clinical and imaging characteristics of rhabdoid tumors of the kidney are similar to those of congenital mesoblastic nephroma, clear cell sarcoma, and Wilms tumor. Therefore, specific diagnosis is usually not possible. One important differentiating point is that clear cell sarcoma of the kidney and rhabdoid tumor of the kidney are invariably unilateral.
The prognosis for patients with a rhabdoid tumor of the kidney is much worse than that for patients with other renal tumors.
Intrarenal neuroblastoma
Abdominal neuroblastomas usually develop in the retroperitoneum. Most arise from the adrenal gland and displace the kidney inferomedially. In rare cases, a neuroblastoma may mimic a Wilms tumor, arising from tissues in the kidney or invading the kidney. To make diagnosis complicated, rare neuroblastomas possess other features more typical of Wilms tumor than of intrarenal neuroblastomas.
Epithelial nephroblastoma
Epithelial nephroblastomatosis, or cystic Wilms tumor, is less malignant than Wilms tumor and may appear cystic or papillary. In its benign form, it gives rise to a multilocular cystic renal disease. Ultrasonographic and CT findings do not help in predicting the degree of malignancy.
Multicystic dysplastic kidney
Multicystic dysplastic kidney is a relatively rare condition. Even so, it is the most common cause of abdominal masses in neonates, leading to 50-65% of renal masses in infancy.
Multicystic dysplastic kidney is a developmental anomaly due to atresia of the upper third of the ureter. In most cases, concomitant atresia affects the renal pelvis and infundibula. The underlying obstruction usually occurs at or before 8-10 weeks of life. Obstruction occurring at a stage later than this gives rise to a relatively rare combination of renal dysplasia and hydronephrosis.
About 33% of patients have contralateral renal anomalies, such as multicystic dysplastic kidney, pelviureteric junction (PUJ) obstruction, hypoplasia, and rotational anomalies. When ureteric duplication is encountered on the contralateral side, one of the ureters may be atretic, with associated segmental renal dysplasia.
Ultrasonography demonstrates a large unilateral renal mass with 10-20 cysts but sometimes as many as 50 cysts of various sizes. Islands of dysplastic renal tissue may be observed between the cysts, but no normal tissue is seen. Antenatal diagnosis is possible. Ultrasonographic findings can help in differentiating multicystic dysplastic kidney and hydronephrosis from other conditions in infants, such as Wilms tumor, neuroblastoma, mesoblastic nephroma, and adrenal hemorrhage.
Autosomal recessive polycystic kidney disease
Autosomal recessive polycystic kidney disease, also known as infantile polycystic disease, is thought to result from dysplasia of the upper collecting system and/or collecting tubules, leading to cysts of various sizes. Microdissection studies have shown fusiform sacculations and cystic diverticula of the distal portions of collecting tubules and collecting ducts, while the proximal collecting tubules are diffusely dilated.
The condition is inherited in an autosomal recessive manner, and it is usually present in infancy or childhood, though it may be diagnosed in utero by means of ultrasonography. Disease severity is usually greatest in patients who present early.
Renal involvement is bilateral, but it may be asymmetric. Autosomal recessive polycystic kidney disease is associated with hepatic fibrosis and ductal hyperplasia, which may cause portal hypertension. In the context of this disease, death is usually caused by renal failure in the youngest children and by hepatic failure in older children.
The disease may be divided according to the patient's age at presentation, as follows:
-
Prenatal form: this form appears in infants, it is rapidly fatal, and it involves 90% of the renal tubules.
-
Infantile form: about 60% of the tubules are involved. The child has uremia but survives longer than those with the prenatal form.
-
Young-childhood form: Affected children present with hypertension and chronic renal failure. Approximately 25% of the renal tubules are involved.
-
Late presentation, juvenile form: symptoms are usually related to hepatic fibrosis, portal hypertension, and GI hemorrhage; in these late cases, small cysts are sometimes seen in the renal cortex.
Ultrasonography shows diffusely enlarged kidneys with a generalized increase in echoes produced by the innumerable fluid–tubular wall interfaces. The renal borders are poorly defined, and corticomedullary differentiation is lost. Echogenicity of the liver is frequently increased.
The peripheral cortex may be spared because it does not have collecting ducts. This feature is found in infants who do not have severe disease and who are likely to survive infancy. However, in severely affected infants, images may show a peripheral sonolucent halo, which may represent markedly dilated ectatic tubules near the renal surface. When high-resolution probes are used, imaging shows a radial array of ectatic and dilated tubules of 1-2 mm in diameter. In utero, the normal combined renal circumference is 27-30% of the abdominal circumference. In infantile polycystic disease, this circumference increases to 60%, a feature that allows for prenatal diagnosis.
Simple renal cysts
The exact etiology of simple renal cysts is uncertain. These lesions may be retention cysts due to obstruction, or they may arise in embryonic rests. Simple renal cysts are uncommon in children, being found in 2-4% of pediatric postmortem examinations. However, their frequency increases with age, and they are found in 50% of adults older than 50 years.
Simple cysts are usually asymptomatic unless they are complicated by hemorrhage or infection. Their mass effect may be large. The cysts arise in a parapelvic position and compress part of the renal collecting system. On sonograms, the cysts are entirely free of echoes, with good sound transmission. They give rise to distal acoustic enhancement. The cyst has a smooth outline without a demonstrable wall. Small cysts may appear echo-free only when they are in the focal zone of the ultrasound beam because of partial-volume affects. Cysts smaller than 3 mm in diameter cannot be identified in the parenchyma.
Ultrasonography is more accurate than CT for visualizing the internal septae and for demonstrating the internal morphologic features of the cyst. If the nature of a cyst identified during sonography is in doubt, follow-up scanning or aspiration of the cyst should be performed. If the appearance is classically that of a simple cyst, no further action is needed.
Multilocular cystic nephroma
Multilocular cystic nephroma is synonymous with multilocular renal cyst, cystic Wilms tumor, hamartoma, cystic adenoma, polycystic nephroblastoma, Perlman tumor, and segmental multicystic kidney. These confusing terms indicate the uncertainty about the etiology and about whether the lesion is dysplastic, neoplastic, or hamartomatous. If a Wilms tumor is noted in the walls of this tumor during histologic analysis, it is often treated as a Wilms tumor.
In terms of its morphologic characteristics, a multilocular renal cyst forms a well-defined, bulky mass arising at the lower pole or between poles of an otherwise normal-looking kidney. The mass has a fibrous capsule, which may contain smooth muscle and cartilage. The tumor mass itself has multiloculated, noncommunicating cysts separated by fibrous tissue. The tumor often protrudes into the renal pelvis, and approximately 50% become calcified.
Some researchers have postulated that focal failure of a ureteral branch to organize this segment of the metanephric blastema gives rise to the appearance just described. Undifferentiated mesenchymal tissue in the various constituents of the fibrous capsule supports the postulate that multilocular cystic nephroma is a type of renal dysplasia.
The ultrasound appearance of a multilocular renal cyst is that of a bulky renal mass with a conglomerate of cysts separated by thick septa protruding into the renal pelvis. Calcification is detected in some masses.
Renal hematoma
Subcapsular hematomas spread around the kidney, giving rise to an echogenic rim. Focal subcapsular hematomas may cause depression of the cortex. As the hematoma resolves, fibrosis may occur, compressing the kidney and resulting in hypertension.
Intrarenal hematomas are more frequently hypoechoic than renal contusions and subcapsular hematomas. Echogenic hematomas may be lost in the central echo complex. Renal hematomas may enlarge; therefore, they should be followed up because they may cause delayed rupture of the kidney.
Beckwith-Wiedemann syndrome
Beckwith-Wiedemann syndrome may be associated with omphalocele (12% of cases) and organomegaly. The incidence of hemihypertrophy, renal anomalies, Wilms tumor, and hepatoblastoma is increased. Antenatal diagnosis is possible.
Heredofamilial features of cystic dysplasia
Cystic renal dysplasia is a component of several familial syndromes. Renal cysts have been described in association with more than 50 syndromes. These include the trisomies, von Hippel-Lindau disease, Jeune syndrome (asphyxiating thoracic dystrophy), Meckel-Gruber syndrome, short rib–polydactyly syndrome, Beckwith-Wiedemann syndrome, and holoprosencephaly.
WAGR syndrome
WAGR syndrome (Wilms tumor, aniridia, genitourinary abnormalities, and a range of developmental delays) is a rare genetic disorder characterized by a de novo deletion of 11p13. [34, 35]
Hydronephrosis
The renal collecting system forms a part of the central echogenic complex, and it is frequently not identifiable as a separate structure. Visualization of the collecting system depends on the rate of urine formation and drainage. Slight dilatation of the collecting system is a common normal finding during diuresis or when the bladder is full. In these cases, the dilatation resolves when the bladder is emptied.
Hydronephrosis is simply dilatation of the renal collecting system. This is not always due to obstruction, and in the converse, obstruction does not always cause hydronephrosis. Hydronephrosis is seen as anechoic fluid in the renal collecting system and pelvis separating the echoes of the central sinus. In long-standing cases, images may show secondary thinning of the renal parenchyma. Dilated calyces lose their sharp, angular margins and become blunted.
When hydronephrosis is considerable, the entire collecting system is outlined as a series of connected fluid-filled channels. When part of the renal collecting system is dilated, the condition may superficially resemble a Wilms tumor on sonographic examination, but close inspection can easily differentiate the 2 conditions.
Renal cell carcinoma
Renal cell carcinoma is a primary epithelial neoplasm that arises from the proximal convoluted tubule. Nearly all cases occur in adults, though the carcinoma has been recorded in children younger than 10 years. Early cases are seen as small, cortical-based masses that enlarge and invade the renal parenchyma. Late cases may cause a classic triad of loin pain, flank mass, and hematuria. Approximately 5% of patients have bilateral tumors, though not usually simultaneously.
Appearances vary widely and depend on the size of the tumor, which may be solid, cystic, or complex. Solid tumors transmit sound waves poorly; therefore, acoustic transmission is unchanged or decreased compared to that of a normal kidney. The most common appearance is increased echogenicity, which corresponds to areas of increased vascularity seen on angiography. Small tumors are particularly echogenic, so small angiomyolipomas cannot be distinguished on sonograms.
Small, hyperechoic renal cell carcinomas may have a hypoechoic rim caused by the tumoral pseudocapsule, and it may contain small, anechoic areas. No true capsule is present, and the tumor margins are irregular. Large tumors tend to be hypoechoic. About 6-20% show evidence of calcification.
Denys-Drash syndrome
Denys-Drash syndrome is a rare entity associated with male pseudohermaphroditism, gonadal dysgenesis, progressive glomerular disease, diffuse mesangial sclerosis, renal failure, and nephroblastoma. [36]
Radiography
Conventional radiography is a noninvasive and economical way to demonstrate lung and bone metastases. Plain radiographs are also used in following up radiation therapy and in looking for pulmonary complications associated with chemotherapy. A plain abdominal radiograph often shows displacement of the abdominal viscera and, in less than 10% of cases, streaky or irregular calcification. Calcification is more apparent on CT than on radiographs. The calcification usually is on the edge of the tumor, whereas the calcification associated with a neuroblastoma is speckled throughout. [15]
Intravenous urography (IVU) shows an intrarenal mass confined within the renal outline (see the image below). The mass is often associated with splaying, distortion, and displacement of the calyces. Three-dimensional assessment can be achieved by using frontal and lateral radiographs. Upper and posterior tumors increase calyceal distortion, as these sites offer little room for exophytic expansion because of their rigid surrounding structures.
 An IVU shows a nonfunctioning left kidney with a suggestion of ill-defined mass in the left loin due to a biopsy-proven Wilms tumor. Note the functioning right duplex renal collecting system. The chest radiograph in the same child shows a lung metastatic deposit (arrow). Images courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
An IVU shows a nonfunctioning left kidney with a suggestion of ill-defined mass in the left loin due to a biopsy-proven Wilms tumor. Note the functioning right duplex renal collecting system. The chest radiograph in the same child shows a lung metastatic deposit (arrow). Images courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
With IVU, an inadequate dose of contrast medium often causes nondiagnostic results, with subsequent errors in diagnosis. Relatively large doses of contrast agent (4 mL/kg) should be used to obtain diagnostic IVUs.
Tumors are commonly large at presentation and often cross the midline. Because large tumors may virtually replace the excretory renal tissue, IVU may show little opacification. This is true in approximately 10% of children with a Wilms tumor, but it does not appear to affect their prognosis.
Tumors at the lower pole and anterior tumors have more room for exophytic growth. Therefore, their associated calyceal deformity is less pronounced than that observed with upper and posterior tumors. A central tumor may cause hydronephrosis and calyceal distortion.
In the developed world, conventional radiography (apart from chest radiography) has a limited role in the workup of Wilms tumor to detect and follow up lung metastases. However, in the developing world, much more reliance may be placed on conventional radiography and perhaps ultrasonography, because these modalities are more readily available. Chest radiography, IVU, and ultrasonography provide the best combination in parts of the world where CT and MRI are not available.
A preoperative imaging protocol that relies predominantly on chest radiography and abdominal ultrasonography does not reduce survival. [37] More sophisticated imaging, particularly CT, is not required in most cases, and it is warranted only when results of chest radiography or ultrasonography are not helpful for resolving relevant management problems. Lung and bone metastases are easily missed on plain radiographs. Moreover, plain radiographs have low specificity.
Computed Tomography
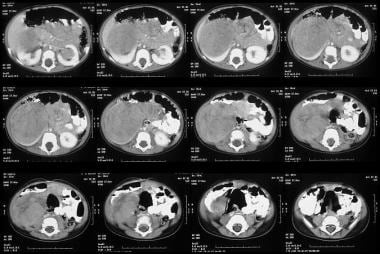
Abdominal CT scanning helps assess the origin of the tumor; lymph node involvement; bilateral renal involvement; and invasion into the renal vascular pedicle, IVC, and right atrium. In addition, scans depict hepatic and distant metastases. CT scan results (see the images below) help confirm that a tumor is in the kidney. Scans often show a surrounding rim of normal renal tissue, distortion of the renal collecting system, and medial displacement of the kidney. By comparison, a neuroblastoma (an important differential diagnosis) rarely distorts the renal collecting system and generally indents or displaces the kidney inferiorly or laterally. Hemorrhagic or cystic areas can be present, but they are not commonly seen. CT demonstrates mixed hypoattenuation with bands of enhancing tissue surrounding cystic and/or necrotic areas. If the rhabdoid variant is present, a CT scan of the head should be obtained to look for evidence of brain metastases. [16, 17, 18, 19, 21, 38, 39]
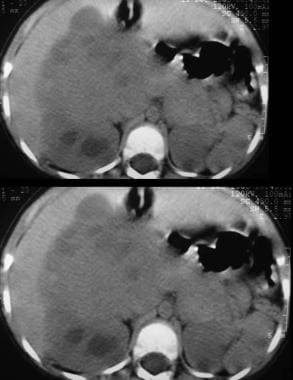
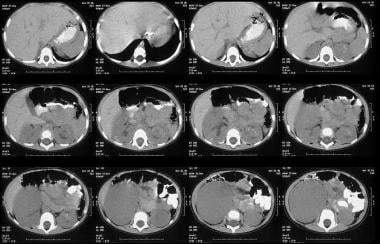 Unenhanced axial CT scans shows a 6 x 8 cm mass low attenuation mass arising from the lower pole of the right kidney and extending into the anterior renal cortex. Also note subtle unrelated medullary left renal nephrocalcinosis.
Unenhanced axial CT scans shows a 6 x 8 cm mass low attenuation mass arising from the lower pole of the right kidney and extending into the anterior renal cortex. Also note subtle unrelated medullary left renal nephrocalcinosis.
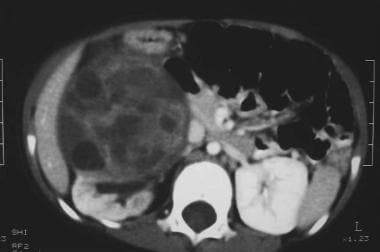
 Enhanced axial CT scan shows a large solid tumor displacing a rim of functioning cortical tissue posteriorly. Note also a second small low attenuation mass in the functioning cortex.
Enhanced axial CT scan shows a large solid tumor displacing a rim of functioning cortical tissue posteriorly. Note also a second small low attenuation mass in the functioning cortex.
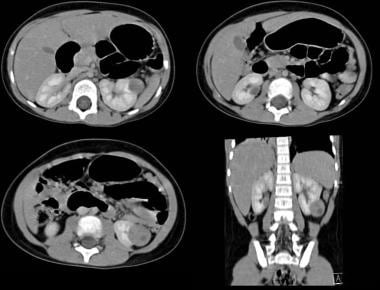 Axial and coronal reconstruction contrast-enhanced CT scan shows a fairly well defined left renal mass with a small central hypodense area due to central necrosis. Image courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
Axial and coronal reconstruction contrast-enhanced CT scan shows a fairly well defined left renal mass with a small central hypodense area due to central necrosis. Image courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
 Unenhanced axial CT scans in the same patient as in the previous image shows a large, solid mass with a heterogeneous mass in the right renal fossa crossing the midline and displacing the liver anteriorly. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
Unenhanced axial CT scans in the same patient as in the previous image shows a large, solid mass with a heterogeneous mass in the right renal fossa crossing the midline and displacing the liver anteriorly. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
 Contrast-enhanced axial CT scan in the same patient as in the previous images shows a large, solid mass with a heterogeneous mass with areas suggestive of necrosis. Note the normal functioning component of the right kidney. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
Contrast-enhanced axial CT scan in the same patient as in the previous images shows a large, solid mass with a heterogeneous mass with areas suggestive of necrosis. Note the normal functioning component of the right kidney. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
With CT and MRI, the use of intravenous contrast material is essential to assess both kidneys. The appropriate dose is 1 mL/0.5 kg body weight, with imaging at 65-70 seconds after the injection to allow the renal vein and the IVC to opacify. The section thickness varies with the capability of the machine being used.
CT helps identify a subgroup of patients with stage I disease who are at increased risk for having a pulmonary relapse. These children receive only single-agent chemotherapy. [38, 39] If findings on chest CT are positive while chest radiographic findings are negative, diagnostic biopsy of the lesions noted on the chest CT scan is recommended. In patients with large tumoral masses, nodal disease may be difficult to distinguish from the primary tumor. CT depicts the most common sites of tumoral spread—namely, the lymph nodes, lungs, and liver. However, CT scanning does not aid in determining whether the tumor can be removed, because this is accurately assessed only at the time of surgery.
Ritchey et al determined the accuracy of preoperative imaging in diagnosing bilateral Wilms tumors. [33] The authors examined case notes of 122 patients with synchronous bilateral Wilms tumors who were enrolled in the fourth National Wilms Tumor Study (NWTS-4). With the exception of one child, all patients underwent abdominal CT, MRI, or ultrasonography. The accuracy of each imaging modality was correlated with the size of the tumors. In 9 patients (7%), bilaterality was missed on the preoperative imaging studies. All but one of the missed lesions were small, 5 were greater than 1 cm, and 3 were 1-3 cm. CT was determined to be more sensitive than ultrasonography in detecting bilaterality. However, there was no single study that was able to depict more than 50% of lesions less than 1 cm in greatest dimension.
Magnetic Resonance Imaging
In general, Wilms tumors have heterogeneously low signal intensity on T1-weighted MRIs and high signal intensity on T2-weighted MRIs. Hyperintense areas on T1-weighted images correspond with hemorrhage. A pseudocapsule is evident on T2-weighted imaging. MRI is not tissue specific. As with other cross-sectional imaging methods, tissue diagnosis is usually required with MRI. Signal intensity on MRIs does not help in distinguishing Wilms tumors from other solid renal tumors. Capsular invasion by a tumor can be missed. [19, 22, 40]
Wilms tumors typically appear inhomogeneous on gadolinium-enhanced images, whereas nephrogenic rests (which are sometimes precursors of Wilms tumors) appear as homogeneous masses. MRA may demonstrate the displacement of the renal vein and IVC, and it may aid in the diagnosis of thrombus of the renal vein of the IVC.
T1 and T2 prolongation is present. Capsular invasion is difficult to predict with imaging. However, CT and MRI are useful in demonstrating extension involving the IVC or tumoral thrombus. In addition, MRI has been used in screening for the nephrogenic rests of nephroblastomatosis and for multicentric Wilms tumors.
MRI may be useful in distinguishing between active nephrogenic rests or Wilms tumor from inactive nephrogenic rests. Information from T2-weighted MRIs is used to make this distinction; active nephrogenic rests and Wilms tumors are both hyperintense, whereas inactive nephrogenic (sclerotic) rests are hypointense. Overall sensitivities for detecting nephrogenic rests are 43% with nonenhanced images and 58% with contrast-enhanced images. Nephrogenic rests admixed with Wilms tumors less than 4 mm in diameter are not identified on MRIs. On gadolinium-enhanced T1-weighted images, Wilms tumors and hyperplastic nephrogenic rests are hypointense relative to normal renal tissue. [41]
Because MRI involves no radiation, this imaging study plays a unique role in the diagnosis and management of pediatric abdominal masses. For instance, whole-body MRI may compete with PET in staging abdominal tumors. Specific advantages of magnetic resonance studies include their usefulness in determining the resectability of tumors with MRI and MRA; the staging of neuroblastomas in the bone marrow, lymph nodes, liver, and spinal canal; evaluating responses of bilateral Wilms tumors and nephroblastomatosis; and detecting pelvic tumors on sagittal sections and detecting peritoneal tumors with contrast enhancement. [40]
Ohnuma et al performed MRI in 126 children with malignant solid tumors, [42] and the investigators judged whether MRI and CT studies yielded equivalent information or whether one study was superior to the other. In 47% of patients, the tumors were better visualized with MRI than with CT. In 43%, MRI was superior to CT for evaluating the local spread of tumor. MRI and CT differed little in the identification of lymph node metastases. Without requiring the injection of intravenous contrast agents, MRI accurately depicted displacement and invasion of the renal vessels by neuroblastoma. MRI was excellent in predicting kidney preservation. Finally, MRI was useful for detecting bone marrow metastases related to neuroblastoma. The coronal plane was the best imaging plane for demonstrating bone marrow involvement in the lower limbs.
Belt et al evaluated the MRI appearances and the clinical utility of MRI in 14 patients with Wilms tumors, and the MRI appearances were correlated with surgical and pathologic findings to assess their accuracy. [43] In all patients, MRI accurately depicted the primary tumor, its renal origin, the tumor margins, and local extension. The margins of the tumors were smooth and well defined in 9 of 12 cases. Local extension and size were accurately assessed. However, because capsular invasion could not be predicted, 4 surgically proven instances of capsular invasion were missed. Metastatic spread into the liver and the IVC was well documented in 4 cases and excluded in 10. MRI was sensitive for identifying lymph node enlargement in 5 of 14 patients, but it was not helpful in predicting the etiology of the enlargement. MRI signal intensity did not aid in distinguishing Wilms tumors from other solid renal tumors.
Ultrasonography
Ultrasonography is an excellent diagnostic modality for evaluating children with a suspected abdominal mass (see the images below). Its major advantages are its lack of radiation, noninvasiveness, and ability to be repeated as needed with no harm to the child. Ultrasonography offers multiplanar capability and can be used to accurately guide biopsy. In addition, 3-dimensional capability is available. The use of ultrasonographic contrast medium is promising. Sedation and a general anesthetic are seldom required, and resolution of both solid and cystic tumors is excellent. Doppler ultrasonography is the imaging modality of choice for evaluating patency of the renal vein and IVC. [14, 15, 44, 28]
 This 6-year-old male child with hematuria was referred for a renal ultrasound scan. The scan shows a 6 x 8 cm solid mass at the lower pole of the right kidney displacing part of the collecting system in a cephalad direction. The mass is of uniform echogenicity with a vague small central hypoechoic area suggestive of tumor necrosis.
This 6-year-old male child with hematuria was referred for a renal ultrasound scan. The scan shows a 6 x 8 cm solid mass at the lower pole of the right kidney displacing part of the collecting system in a cephalad direction. The mass is of uniform echogenicity with a vague small central hypoechoic area suggestive of tumor necrosis.
 Axial US image shows a solid 4.5-cm solid mass anterior cortex, lower pole of the left kidney. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
Axial US image shows a solid 4.5-cm solid mass anterior cortex, lower pole of the left kidney. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
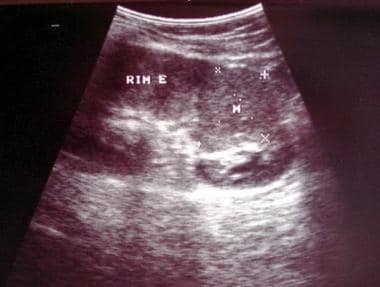 Sagittal US scan of the left kidney (same patient as in the previous image). Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
Sagittal US scan of the left kidney (same patient as in the previous image). Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
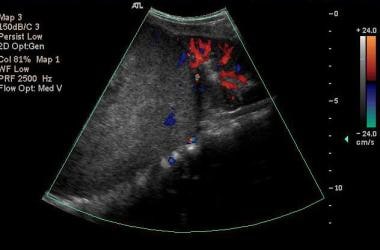 Color Doppler US scan of the right kidney in a sagittal plane shows a 13-cm upper-pole, mainly solid tumor with a heterogeneous echo pattern displacing the functioning component of the kidney inferiorly.
Color Doppler US scan of the right kidney in a sagittal plane shows a 13-cm upper-pole, mainly solid tumor with a heterogeneous echo pattern displacing the functioning component of the kidney inferiorly.
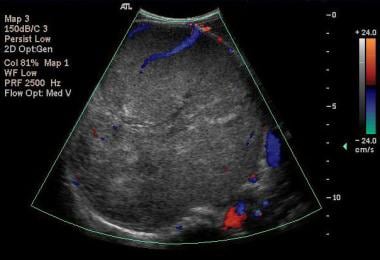 Color Doppler US scan of the right kidney in an axial plane shows a 13-cm upper-pole, mainly solid tumor with a heterogeneous echo pattern displacing the vessels and crossing the midline (same patient as in previous image). Images courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
Color Doppler US scan of the right kidney in an axial plane shows a 13-cm upper-pole, mainly solid tumor with a heterogeneous echo pattern displacing the vessels and crossing the midline (same patient as in previous image). Images courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
The initial diagnosis of a suspected renal or abdominal mass, with a possible renal vein or IVC thrombus in children is usually confirmed by using ultrasonography. Sonograms provide information regarding the liver and the unaffected kidney and confirms an intrarenal location of the tumor. Ultrasonography is also an excellent screening tool for syndromes associated with Wilms tumors, such as nephroblastomatosis, hemihypertrophy, horseshoe kidney, Beckwith-Wiedemann syndrome, chromosomal abnormalities, Denys-Drash syndrome, and Perlmann syndrome. Renal involvement is usually present, but rare extrarenal variants have also been reported, both within the abdomen and at distal extra-abdominal sites.
Approximately 10% of children with Beckwith-Wiedemann syndrome develop cancer. However, the risk is high enough to warrant screening for cancer. The incidence of cancer is age dependent, with the risk being high in the first 4 years, low at 5-10 years, and near the baseline for the general population by 10 years of age.
The major reason for cancer screening is because early identification improves survival and decreases morbidity associated with treatment. For screening, ultrasonography is performed every 3 months.
Screening does increase the number of children in whom the tumor can be removed while part of the kidney is spared. The current recommendation is the performance of ultrasonography every 3 months until 6-8 years of age. For children with Beckwith-Wiedemann syndrome and hemihypertrophy, the adrenal glands and liver should be evaluated in addition to the kidney because they are also at risk for developing hepatoblastoma and neuroblastoma.
Egeler and associates showed that a preoperative imaging protocol based on predominantly chest radiography and abdominal ultrasonography does not reduce the survival of patients with Wilms tumors. [37]
Skoldenberg et al retrospectively assessed the safety, sensitivity, and specificity of biopsy done with an ultrasonography-guided cutting needle (needle diameter, 1.2 mm) in 28 children with renal tumors. All Wilms tumors were correctly diagnosed. The overall sensitivity was 76%. Use of the ultrasonography-guided cutting needle proved to be a safe procedure, and it was not associated with recurrence along the needle tract or other serious complications. [45]
Sklair-Levy et al performed 69 percutaneous image-guided needle biopsy procedures in 57 children. Their data suggested that image-guided needle biopsy is an excellent tool for diagnosing solid tumors in the pediatric population. Negative results should be considered nondiagnostic and followed up with excisional surgical biopsy when the clinical suspicion of malignancy is high. [46]
In a study of ultrasound and laboratory findings in Wilms tumor survivors with a solitary kidney, signs of kidney damage were seen in 22 of 53 patients (41.5%) on ultrasonography. The most frequently detected abnormalities were hyperechoic rings around renal pyramids (28.3% of patients). Hypertrophy of the solitary kidney occurred in 71,7% of cases. [28]
General ultrasound appearance
Ultrasonographic findings may be summarized as follows:
-
The mass is solid at presentation and usually >10 cm.
-
Echogenicity is variable; the lesions are generally echogenic, but cysts within the tumor contribute to the complexity; these cysts may due to tumor necrosis, deposits of mucin, or simply trapped calyces.
-
Calcification is uncommon; when present, it is usually irregular and amorphous.
-
Intense echogenic foci in the tumor may be related to fat deposition.
-
The IVC should be imaged to the level of the right atrium to assess for extension of the tumor; IVC-related tumor extension may be seen on real-time ultrasonography and, if necessary, confirmed with duplex or color Doppler imaging; tumors on the right side may compress the IVC and make it difficult to visualize, though the IVC may be patent.
-
The contralateral kidney should always be examined because 6% of tumors are bilateral at the time of presentation and because their detection alters primary therapy.
-
Anomalies that could alter surgery (eg, contralateral renal agenesis, horseshoe kidney, pelviureteric [PUJ] obstruction) must be excluded.
-
An imaging review indicates that the addition of other imaging studies to plain abdominal radiography and ultrasonography does not alter patients' outcomes.
Neonatal renal masses
Findings associated with a unilateral mass are as follows [44] :
-
Multicystic kidney (15%)
-
Ureteropelvic (UPJ) obstruction/hydronephrosis (25%)
-
Hydronephrotic upper moiety
-
Renal vein thrombosis
-
Mesoblastic nephroma
-
Wilms teratoma (rare)
Findings associated with bilateral masses are as follows [44] :
-
Hydronephrosis
-
Polycystic disease
-
Multicystic kidney with contralateral hydronephrosis
-
Nephroblastomatosis
-
Bilateral multicystic kidneys
-
Renal masses in older children
Findings associated with a solitary mass are as follows [44] :
-
Wilms tumor
-
Multilocular cystic nephroma
-
Focal hydronephrosis
-
Traumatic cyst
-
Renal abscess
-
Renal cell carcinoma
-
Clear cell sarcoma
-
Rhabdoid tumor of the kidney
-
Intrarenal neuroblastoma
Findings associated with multiple masses are as follows [44] :
-
Nephroblastomatosis
-
Multiple Wilms tumors
-
Angiomyolipoma
-
Lymphoma/leukemia
-
Adult polycystic disease
-
Abscesses
Findings associated with pediatric cystic renal masses are as follows [44] :
-
Hydronephrosis
-
Duplication with obstructed moiety
-
Multicystic dysplastic kidney
-
Adult polycystic disease
-
Cystic Wilms tumors
-
Multiseptate urinoma
-
Multilocular cystic nephroma
-
Hematoma
-
Abscess
Findings associated with pediatric solid renal tumors are as follows [44] :
-
Wilms tumor
-
Hamartoma
-
Nephroblastomatosis
-
Mesoblastic nephroma
-
Infantile polycystic disease
-
Rhabdomyosarcoma
-
Leiomyosarcoma
-
Angiomyolipoma
-
Hemangioma
-
Hemangiopericytoma
-
Mucosal epithelial tumors
-
Clear cell sarcoma
-
Renal cell carcinoma
-
Metastases
-
Lymphoma
-
Leukemia
-
Rhabdoid tumor of the kidney
-
Pseudotumors
Nuclear Imaging
Bone scintigraphy is a sensitive means for detecting bony metastases. Bone scanning is necessary in children with clear cell sarcoma. If the clear cell variant of Wilms tumor is present on postsurgical pathologic analysis, a bone scan is obtained to look for spread to bone. Spread to the bone is unusual for other types of Wilms tumors. Bony metastases of osteoblastic Wilms tumors may be photon deficient. If small, they may be missed on isotopic bone scans. [20]
Radionuclide studies performed with technetium-99m DMSA may be indicated to assess the volume of functioning renal tissues and thus guide what tissue may be preserved when nephron-preserving surgery of bilateral tumors is being contemplated. Isotopic renography is a sensitive technique for assessing renal function. Gallium-67 citrate appears to be a highly valuable radionuclide that may be used to differentiate an infectious process in the kidney from nephroblastomatosis. [47]
Wilms tumors appear to concentrate 18F-fluorodeoxyglucose (FDG), a feature that might prove clinically useful. [48, 49] Shulkin et al examined 3 patients with known or suspected Wilms tumor who underwent imaging with FDG-PET, [50] and in all 3 patients the results of the PET scans influenced the therapeutic decisions. The investigators concluded that FDG-PET scanning may be useful for managing Wilms tumors in selected patients.
Nuclear medicine plays an important and increasing role in the management of solid childhood tumors. It is also helpful in managing the complications of cancer treatment, such as the infections often associated with immunosuppression in oncology patients.
Scintigraphy is a complementary investigation to other radiologic techniques and adds a functional element to anatomic cross-sectional imaging. Scintigraphy is used in the initial diagnosis, staging, and assessment of tumoral responses to treatment. It also aids in detecting recurrence and in diagnosing complications. Scintigraphy has a role in the differential diagnosis of Wilms tumors, in their staging, and in imaging therapy-related complications.
Lepanto et al examined 20 pediatric patients with malignant disease using whole-body gallium scans. [51] Of 50 sites studied, true-positive results were found in 17%, and false-negative results were noted in 58%. The method served best in patients with Hodgkin disease, in whom the true-positive rate was 64%. In no instance did the results of gallium scanning affect the clinical care of a patient.
Angiography
With the availability of noninvasive procedures such as Doppler imaging and MRA, angiography is now rarely used in the diagnosis and staging of Wilms tumors. Tortuous tumor vessels of a spider-leg nature may be seen, and large tumors may have collateral circulation from adjacent lumber vessels. The extent of neovascularity helps in planning the preoperative assessment of the tumor.
Rosch et al used sequential angiography to evaluate 6 children with solid abdominal malignancies with sequential angiography [52] and found that angiography was valuable for demonstrating the tumor, as well as its location, extent, vascular characteristics, regression, and recurrence. Wilms tumors and neuroblastomas responded well to irradiation and chemotherapy by substantially shrinking, with regression or disappearance of its neovasculature. Resection of the tumors revealed that these effects were due to tumor necrosis, hemorrhage, and/or cystic degeneration.
Albert and Petty reported a case of spontaneous renal hemorrhage [53] that appeared to be polyarteritis nodosa on arteriography but turned out to be a malignant process. This case stresses the overlapping angiographic appearances of the 2 disease processes and emphasizes the need for tissue diagnosis because radiographic findings may not be as specific as reported.
Questions & Answers
Overview
Which modalities are preferred for Wilms tumor imaging?
When is a chest CT indicated in Wilms tumor imaging?
What are the objectives of Wilms tumor staging?
What is the role of invasive imaging in the workup of Wilms tumor?
What is the role of nuclear imaging in the workup of Wilms tumor?
What is the prognosis of Wilms tumor?
How is renal blastema and/or nephroblastomatosis differentiated from Wilms tumor on imaging?
How is mesoblastic nephroma differentiated from Wilms tumor on imaging?
How is clear cell sarcoma differentiated from Wilms tumor on imaging?
How is rhabdoid tumor of the kidney differentiated from Wilms tumor on imaging?
How is intrarenal neuroblastoma differentiated from Wilms tumor on imaging?
How is epithelial nephroblastoma differentiated from Wilms tumor on imaging?
How is multicystic dysplastic kidney differentiated from Wilms tumor on imaging?
How is autosomal recessive polycystic kidney disease differentiated from Wilms tumor on imaging?
How are simple renal cysts differentiated from Wilms tumor on imaging?
How is multilocular cystic nephroma differentiated from Wilms tumor on imaging?
How is renal hematoma differentiated from Wilms tumor on imaging?
How is Beckwith-Wiedemann syndrome differentiated from Wilms tumor on imaging?
What is WAGR syndrome (Wilms tumor, aniridia, genitourinary abnormalities, mental retardation)?
How is hydronephrosis differentiated from Wilms tumor on imaging?
How is renal cell carcinoma differentiated from Wilms tumor on imaging?
How is Denys-Drash syndrome differentiated from Wilms tumor?
Which radiography findings are characteristic of Wilms tumor?
What is the role of CT in Wilms tumor imaging?
What is the role of MRI in Wilms tumor imaging?
What is the role of ultrasonography in Wilms tumor imaging?
Which ultrasonographic findings are characteristic of Wilms tumor?
Which ultrasonographic findings are associated with unilateral renal mass in neonates?
Which ultrasonographic findings are associated with bilateral masses in neonates?
Which ultrasonographic findings are associated with a solitary mass in neonates?
Which ultrasonographic findings are associated with multiple masses in neonates?
Which ultrasonographic findings are associated with pediatric cystic renal masses?
What are ultrasonography findings are associated with pediatric solid renal tumors?
What is the role of bone scintigraphy in Wilms tumor imaging?
What is the role of angiography in Wilms tumor imaging?
-
This 6-year-old male child with hematuria was referred for a renal ultrasound scan. The scan shows a 6 x 8 cm solid mass at the lower pole of the right kidney displacing part of the collecting system in a cephalad direction. The mass is of uniform echogenicity with a vague small central hypoechoic area suggestive of tumor necrosis.
-
Unenhanced axial CT scans shows a 6 x 8 cm mass low attenuation mass arising from the lower pole of the right kidney and extending into the anterior renal cortex. Also note subtle unrelated medullary left renal nephrocalcinosis.
-
Enhanced axial CT scan shows a large solid tumor displacing a rim of functioning cortical tissue posteriorly. Note also a second small low attenuation mass in the functioning cortex.
-
An IVU shows a nonfunctioning left kidney with a suggestion of ill-defined mass in the left loin due to a biopsy-proven Wilms tumor. Note the functioning right duplex renal collecting system. The chest radiograph in the same child shows a lung metastatic deposit (arrow). Images courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
-
Axial US image shows a solid 4.5-cm solid mass anterior cortex, lower pole of the left kidney. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
-
Sagittal US scan of the left kidney (same patient as in the previous image). Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
-
Axial and coronal reconstruction contrast-enhanced CT scan shows a fairly well defined left renal mass with a small central hypodense area due to central necrosis. Image courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
-
Color Doppler US scan of the right kidney in a sagittal plane shows a 13-cm upper-pole, mainly solid tumor with a heterogeneous echo pattern displacing the functioning component of the kidney inferiorly.
-
Color Doppler US scan of the right kidney in an axial plane shows a 13-cm upper-pole, mainly solid tumor with a heterogeneous echo pattern displacing the vessels and crossing the midline (same patient as in previous image). Images courtesy Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
-
Unenhanced axial CT scans in the same patient as in the previous image shows a large, solid mass with a heterogeneous mass in the right renal fossa crossing the midline and displacing the liver anteriorly. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com
-
Contrast-enhanced axial CT scan in the same patient as in the previous images shows a large, solid mass with a heterogeneous mass with areas suggestive of necrosis. Note the normal functioning component of the right kidney. Image courtesy of Dr. Pedro Daltro and Dr. Edson Marchiori, Port Allegre, Brazil. edmarchiori@gmail.com








