Practice Essentials
Pancreas divisum (PD), the most common congenital variant of the pancreatic anatomy, occurs when the ductal systems of the ventral and dorsal pancreatic ducts fail to fuse. As a result of nonunion of the ducts, a major portion of pancreatic exocrine secretions enter the duodenum via the dorsal duct and minor papilla. Generally, it has been accepted that a relative obstruction to pancreatic exocrine secretory flow through the minor duct and minor papilla can result in pancreatitis in a small number of patients with pancreas divisum (with stenotic minor papilla).
The cause of PD is the failure of fusion of the 2 ductal systems (ventral bud [duct of Wirsung] and the dorsal bud [duct of Santorini]), with the result being classic (or complete), incomplete, or reverse pancreas divisum. Magnetic resonance cholangiopancreatography (MRCP), MRCP after secretin stimulation (S-MRCP), and endoscopic ultrasonography (EUS) have all been shown to be reliable in making the diagnosis of pancreas divisum. Contrast-enhanced computed tomography (CT) may help identify variations in pancreatic ductal anatomy but has a low sensitivity (50–60%), especially if the ductal system is obscured by pancreatic disease such as pancreatitis. Endoscopic retrograde cholangiopancreatography (ERCP) was historically the test of choice for making a diagnosis of pancreas divisum, but it is expensive and invasive, with a reported complication rate of 5%. [1, 2, 3, 4, 5, 6]
Recurrent acute pancreatitis, chronic pancreatitis, and chronic abdominal pain are the main clinical presentations of patients with pancreas divisum. [2] In patients with complete or classic PD, the ventral duct system is short and opens into the major papilla, while the longer dorsal duct system opens into the minor papilla, with no communication between the 2 ducts. Incomplete or partial PD is defined as the communication of the dorsal and ventral ducts via a tiny branch. Reverse pancreas divisum is when the accessory duct of Santorini does not communicate with the main pancreatic duct, leading to a small isolated component of the dorsal pancreas. [2, 1]
(See the images below.)
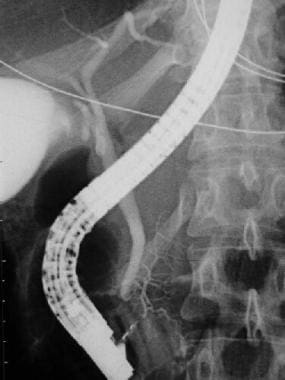 Endoscopic retrograde cholangiopancreatography (ERCP) of a patient with pancreas divisum. A contrast injection following cannulation of the ampulla of Vater demonstrates filling of the common bile duct and a small pancreatic duct of Wirsung, which drains the pancreatic head.
Endoscopic retrograde cholangiopancreatography (ERCP) of a patient with pancreas divisum. A contrast injection following cannulation of the ampulla of Vater demonstrates filling of the common bile duct and a small pancreatic duct of Wirsung, which drains the pancreatic head.
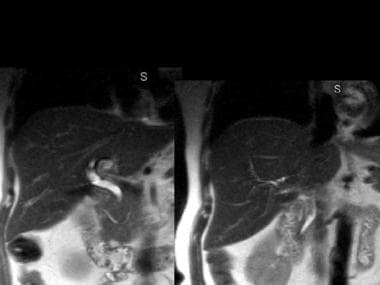 Magnetic resonance cholangiopancreatography (MRCP) of a patient with pancreas divisum. The pancreatic duct of Santorini and the common bile duct are visualized. (Courtesy of Glenn Krinsky, MD)
Magnetic resonance cholangiopancreatography (MRCP) of a patient with pancreas divisum. The pancreatic duct of Santorini and the common bile duct are visualized. (Courtesy of Glenn Krinsky, MD)
Preferred examination
ERCP was historically the test of choice for making a diagnosis of pancreas divisum, but it is expensive and invasive, with a reported complication rate of 5%. Occasionally, this anomaly can be depicted with computed tomography (CT) scanning, but CT has a low sensitivity and requires thin slices. Endoscopic retrograde cholangiopancreatography (ERCP) is used for therapeutic intervention in patients with pancreas divisum. [5]
Contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) has been reported to have a sensitivity of 50-70%. Studies of secretin-enhanced MRCP have shown a sensitivity of up to 86%, with a specificity of 97–99%. MRCP makes use of heavily T2-weighted pulse sequences to depict the fluid-containing pancreaticobiliary duct system. For a complete abdominal workup, MRCP is combined with other T2-weighted imaging sequences such as half Fourier acquisition single shot turbo spin echo sequences (HASTE) and inversion recovery turbo spin echo sequences (TIRM). [2, 1, 3, 4, 5]
Endoscopic ultrasound (EUS) has also been reported to have a high diagnostic accuracy for PD, with a sensitivity of 87-95%, with secretin enhancement (S-EUS) offering marginal benefit. [2, 1, 3]
Systematic reviews suggest S-MRCP has a superior accuracy to EUS and is the preferred diagnostic modality of choice. [2, 1, 3]
Radiography
ERCP was historically the test of choice for making a diagnosis of pancreas divisum, but it is expensive and invasive, with a reported complication rate of 5%. Occasionally, this anomaly can be depicted with computed tomography (CT) scanning, but CT has a low sensitivity and requires thin slices. ERCP is used for therapeutic intervention in patients with pancreas divisum.
(See the images below.)
 Endoscopic retrograde cholangiopancreatography (ERCP) of a patient with pancreas divisum. A contrast injection following cannulation of the ampulla of Vater demonstrates filling of the common bile duct and a small pancreatic duct of Wirsung, which drains the pancreatic head.
Endoscopic retrograde cholangiopancreatography (ERCP) of a patient with pancreas divisum. A contrast injection following cannulation of the ampulla of Vater demonstrates filling of the common bile duct and a small pancreatic duct of Wirsung, which drains the pancreatic head.
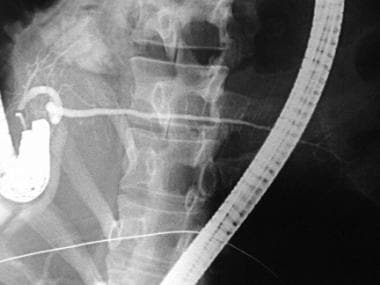 ERCP of a patient with pancreas divisum. Injection of contrast following cannulation of the minor ampulla demonstrates filling of a separate, larger duct of Santorini, which drains the entire pancreatic body and tail.
ERCP of a patient with pancreas divisum. Injection of contrast following cannulation of the minor ampulla demonstrates filling of a separate, larger duct of Santorini, which drains the entire pancreatic body and tail.
Criteria for the diagnosis of pancreas divisum on ERCP are as follows (see the images below):
-
Cannulation of the ampulla of Vater, which allows filling of a short (10-60 mm) and thin (2-mm diameter) main pancreatic duct, located in a posterior position
-
Cannulation of the accessory papilla (allows filling of a larger duct, 2-4 mm in diameter), which drains almost the entire pancreas from the tail to the anterior part of the head
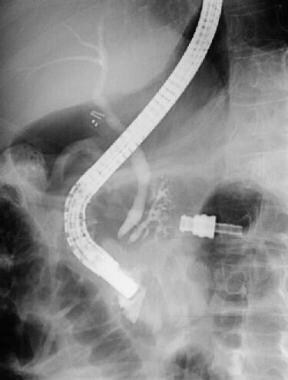 ERCP of a patient with pancreas divisum. Injection of a contrast agent following endoscopic cannulation of the major ampulla results in filling of the common bile duct and a short pancreatic duct of Wirsung. Contrast filling of pancreatic acini in the pancreatic head is related to the pressure of the contrast injection. Surgical clips are seen from a prior cholecystectomy.
ERCP of a patient with pancreas divisum. Injection of a contrast agent following endoscopic cannulation of the major ampulla results in filling of the common bile duct and a short pancreatic duct of Wirsung. Contrast filling of pancreatic acini in the pancreatic head is related to the pressure of the contrast injection. Surgical clips are seen from a prior cholecystectomy.
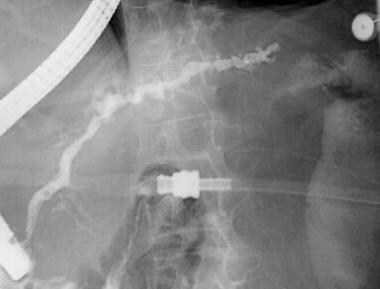 ERCP of a patient with pancreas divisum. Injection of a contrast agent following cannulation of the minor ampulla results in filling of a separate pancreatic duct of Santorini, with dilation, beading, and stricture of the main duct and with dilation and clubbing of the secondary ducts. Findings are consistent with the changes of chronic pancreatitis.
ERCP of a patient with pancreas divisum. Injection of a contrast agent following cannulation of the minor ampulla results in filling of a separate pancreatic duct of Santorini, with dilation, beading, and stricture of the main duct and with dilation and clubbing of the secondary ducts. Findings are consistent with the changes of chronic pancreatitis.
Degree of confidence
Endoscopic cannulation of the accessory papilla is considered technically difficult. Because of the small size of the accessory papilla, it is difficult to recognize and localize. The overall success rate of cannulation depends on the experience of the endoscopic team, technical improvements in endoscopes and catheters, and the interest in precisely documenting ductal morphology. Contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) has been reported to have a sensitivity of 50-70%. Studies of secretin-enhanced MRCP have shown a sensitivity of up to 86%, with a specificity of 97–99%.
Computed Tomography
CT is frequently used to evaluate patients with chronic pancreatitis or chronic abdominal pain. Images are analyzed for glandular size, contour, and focal alteration in attenuation. Single or multislice helical CT with 1-3 mm collimation, overlapping reconstructions, and the use of water as a negative contrast agent provide for high-quality images amenable to three-dimensional (3D) reformations. While demonstration of ductal anatomy by CT is more difficult than demonstration of gross glandular architecture, use of meticulous technique, minimum-intensity projection algorithms, and curved reformations can often show the pancreatic duct throughout its entire course.
CT criterion for the diagnosis of pancreas divisum is visualization of the nonunion of the dorsal and ventral ducts directly joining the common bile duct. Although it is possible to confuse a patent accessory duct of Santorini with divisum, in the normal pancreas, a vertical segment of the main pancreatic duct caudal to the accessory duct is usually seen on its way to the ampulla of Vater. In divisum, this segment of the duct is not present. In addition, an accessory duct should not be confused with a ventral duct, since the accessory duct does not join the bile duct. See the images below.
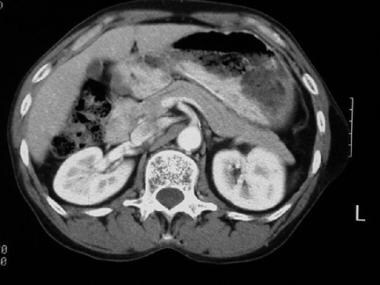 Computed tomography (CT) scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively.
Computed tomography (CT) scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively.
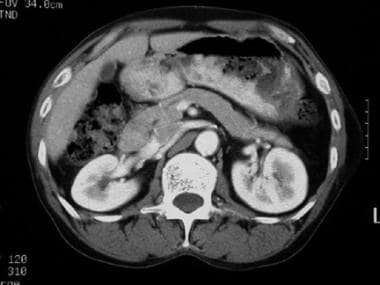 CT scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively.
CT scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively.
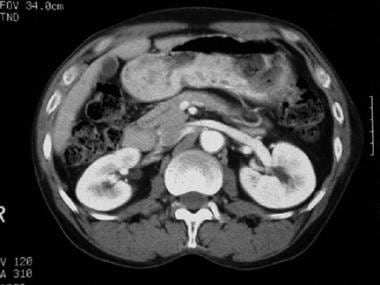 CT scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively. Note that the pancreatic duct of Santorini is located anterior to the intrapancreatic portion of the common bile duct.
CT scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively. Note that the pancreatic duct of Santorini is located anterior to the intrapancreatic portion of the common bile duct.
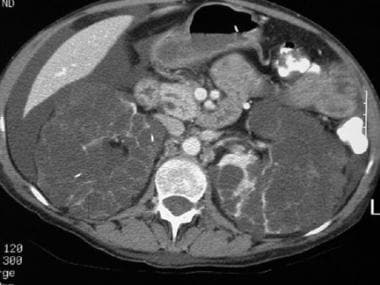 CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note that the pancreatic duct of Santorini is located anterior to the common bile duct and the duct of Wirsung in the pancreatic head. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note that the pancreatic duct of Santorini is located anterior to the common bile duct and the duct of Wirsung in the pancreatic head. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
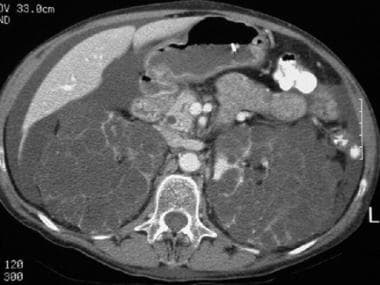 CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note that the pancreatic duct of Santorini is located anterior to the common bile duct and duct of Wirsung in the pancreatic head and neck. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note that the pancreatic duct of Santorini is located anterior to the common bile duct and duct of Wirsung in the pancreatic head and neck. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
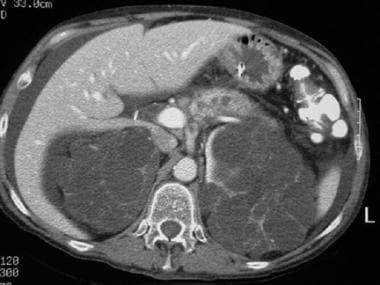 CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note the irregular dilatation of the duct of Santorini in the pancreatic body and tail. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note the irregular dilatation of the duct of Santorini in the pancreatic body and tail. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
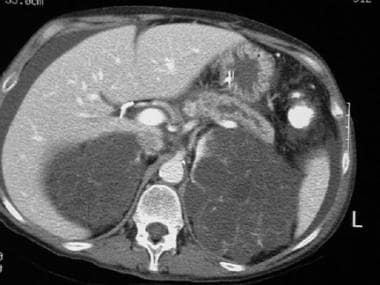 CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate ducts of Santorini and Wirsung. Note the irregular dilatation of the duct of Santorini in the pancreatic body and tail. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate ducts of Santorini and Wirsung. Note the irregular dilatation of the duct of Santorini in the pancreatic body and tail. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
Additional evidence of pancreas divisum on dynamic thin-section CT is the visualization of a fat-attenuation cleft separating the pancreatic head from the pancreatic body. This cleft runs obliquely through the section and acts as a boundary between the 2 distinct pancreatic moieties visible at the same craniocaudal level. Fatty infiltration of the dorsal portion of the gland can also be observed with a normal ventral portion.
False positives/negatives
Complete pancreatic ductal anatomy and the presence of separate dorsal and ventral pancreatic moieties are seen only in a minority of patients using conventional CT. Zeman et al [7] were able to definitively identify separate dorsal and ventral ducts in only 5 of 12 patients with known pancreas divisum. Thin-section multislice helical CT may be more accurate, but no studies using this technique have been reported.
Magnetic Resonance Imaging
Contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) has been reported to have a sensitivity of 50-70%. Studies of secretin-enhanced MRCP have shown a sensitivity of up to 86%, with a specificity of 97–99%. [2, 1, 3] MRCP is a noninvasive imaging modality that accurately and simultaneously depicts morphologic abnormalities of the biliary and the pancreatic ducts and parenchymal structures. [8, 9, 10, 11, 12, 13] Bret et al were able to identify pancreas divisum in 6 of 114 MRCP examinations using a body coil and 19 of 154 MRCP examinations using a torso coil. [14]
Exogenous administration of secretin stimulates the secretion of fluids and bicarbonates by the exocrine pancreas, with a consequent increase in the volume of fluid inside the pancreatic ducts. This increase in fluid content is used to improve the visualization of pancreatic ductal anatomy on MRCP. Nonfusion of ventral and dorsal pancreatic ducts in pancreas divisum can be recognized more readily after secretin-stimulated MRCP. The amount of fluid secreted into the duodenum can also be quantified to assess the degree of pancreatic exocrine reserve in patients with chronic pancreatitis related to pancreatic divisum. [11, 15]
Manfredi et al identified pancreas divisum in 7% of patients (6 of 84) by MRCP before secretin administration and in 14% of patients (12 of 84) following secretin administration. Development of half-Fourier pulse sequences and phased-array surface coils enables the acquisition of high-quality images during breath holding, with a high signal-to-noise ratio. [16]
Parameters that can be assessed before and after administration of secretin in MRCP include the following:
-
Complete ductal anatomy with presence or absence of pancreas divisum
-
Number of segments of main pancreatic ducts visualized (head, body, tail)
-
Visualization of side branches
-
Presence of ductal narrowing
-
Intraluminal filling defects
Abnormalities indicative of chronic pancreatitis secondary to minor papilla stenosis in patients with pancreas divisum can be seen more frequently following secretin administration.
In the series by Bret et al, ERCP was performed following MRCP in fewer than half of the patients, but the sensitivity and specificity of MRCP for pancreas divisum was 100% in this group. [14]
Ultrasonography
Secretin-stimulated ultrasonography (US) is a noninvasive test that shows great promise and objectivity and has been popularized by Warshaw et al. [17] The test involves sequential ultrasonographic measurement of the pancreatic duct size following intravenous administration of secretin. A significant limitation of this test is that the pancreas cannot be reliably visualized in all patients because of body habitus, overlapping bowel gas, or pain.
Although secretin-stimulated US in pancreas divisum may not demonstrate the anomalous ductal anatomy, it may provide evidence of stenosis in patients with acute recurrent pancreatitis or chronic pancreatitis and predict which patients may respond to surgical or endoscopic therapy.
Secretin-stimulated US is 78% sensitive in detecting surgically proven pancreatic duct stenosis.
Systematic reviews suggest S-MRCP has a superior accuracy to EUS and is the preferred diagnostic modality of choice. [2, 1, 3]
-
Endoscopic retrograde cholangiopancreatography (ERCP) of a patient with pancreas divisum. A contrast injection following cannulation of the ampulla of Vater demonstrates filling of the common bile duct and a small pancreatic duct of Wirsung, which drains the pancreatic head.
-
ERCP of a patient with pancreas divisum. Injection of contrast following cannulation of the minor ampulla demonstrates filling of a separate, larger duct of Santorini, which drains the entire pancreatic body and tail.
-
Computed tomography (CT) scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively.
-
CT scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively.
-
CT scan of a patient with pancreas divisum. Sequential axial CT images demonstrate separate pancreatic ducts of Santorini and Wirsung to the region of the minor and major ampulla, respectively. Note that the pancreatic duct of Santorini is located anterior to the intrapancreatic portion of the common bile duct.
-
CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note that the pancreatic duct of Santorini is located anterior to the common bile duct and the duct of Wirsung in the pancreatic head. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
-
CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note that the pancreatic duct of Santorini is located anterior to the common bile duct and duct of Wirsung in the pancreatic head and neck. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
-
CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate pancreatic ducts of Santorini and Wirsung. Note the irregular dilatation of the duct of Santorini in the pancreatic body and tail. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
-
CT scan of a patient with pancreas divisum. Sequential axial CT images in a patient with a history of acute recurrent pancreatitis demonstrate separate ducts of Santorini and Wirsung. Note the irregular dilatation of the duct of Santorini in the pancreatic body and tail. Incidental note is made of autosomal dominant polycystic kidney disease in this patient.
-
ERCP of a patient with pancreas divisum. Injection of a contrast agent following endoscopic cannulation of the major ampulla results in filling of the common bile duct and a short pancreatic duct of Wirsung. Contrast filling of pancreatic acini in the pancreatic head is related to the pressure of the contrast injection. Surgical clips are seen from a prior cholecystectomy.
-
ERCP of a patient with pancreas divisum. Injection of a contrast agent following cannulation of the minor ampulla results in filling of a separate pancreatic duct of Santorini, with dilation, beading, and stricture of the main duct and with dilation and clubbing of the secondary ducts. Findings are consistent with the changes of chronic pancreatitis.
-
ERCP of a patient with pancreas divisum. This patient was treated with a minor ampulla sphincterotomy and pancreatic duct stent placement.
-
Magnetic resonance cholangiopancreatography (MRCP) of a patient with pancreas divisum. The pancreatic duct of Santorini and the common bile duct are visualized. (Courtesy of Glenn Krinsky, MD)





