Background
The occurrence of an immunologically mediated and injurious set of reactions by cells genetically disparate to their host, otherwise known as graft versus host disease (GVHD), is a phenomenon that has been described as the age of bone marrow and solid organ transplantation has emerged. In 1962, Barnes and Loutit first described GVHD in mice. [1] Simonsen introduced the term graft-versus-host reaction in the 1960s to describe the direction of the immunological damage caused by introduction of immunologically competent cells into an immunocompromised host. [2] In 1966, Billingham proposed 3 conditions required for the development of GVHD, as follows: (1) the graft must contain immunologically competent cells, (2) the host must possess important transplant alloantigens that are lacking in the donor graft so that the host appears foreign to the graft, and (3) the host itself must be incapable of mounting an effective immunologic reaction against the graft. [3] See the image below.
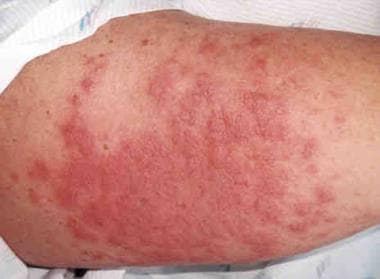 Autologous graft versus host disease (GVHD) involving the skin of a patient's arm shortly after showing signs of engraftment after an autologous peripheral blood stem cell transplant for ovarian cancer. Image courtesy of Romeo A. Mandanas, MD, FACP.
Autologous graft versus host disease (GVHD) involving the skin of a patient's arm shortly after showing signs of engraftment after an autologous peripheral blood stem cell transplant for ovarian cancer. Image courtesy of Romeo A. Mandanas, MD, FACP.
According to the accepted definition, the immunologic assault itself and its consequences are referred to as GVHD. In both experimental and clinical scenarios, acute GVHD, as shown below, describes a syndrome consisting of dermatitis, enteritis, and hepatitis occurring within the first 100 days, but typically within 30-40 days, following a bone marrow transplant (BMT). Chronic GVHD usually develops after 100 days and describes an autoimmunelike syndrome consisting of impairment of multiple organs or organ systems. [4] However, the timing of GVHD occurrence to define the acute versus chronic is arbitrary.
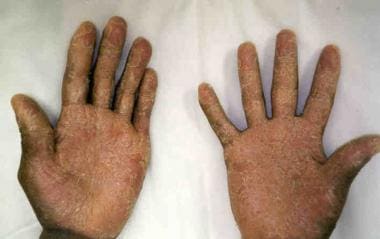 Acute graft versus host disease (GVHD) involving desquamating skin lesions in a patient following allogeneic bone marrow transplantation for myelodysplasia. Image courtesy of Romeo A. Mandanas, MD, FACP.
Acute graft versus host disease (GVHD) involving desquamating skin lesions in a patient following allogeneic bone marrow transplantation for myelodysplasia. Image courtesy of Romeo A. Mandanas, MD, FACP.
With the advances of transplant practice, the clinical manifestations are now better defined than timing alone. The National Institutes of Health (NIH) published consensus criteria for the diagnosis of GVHD and proposed 2 subcategories for acute GVHD (classic acute and late acute) and chronic GVHD (classic chronic and overlap syndrome), taking an organ-functional impact into the account. [5] However, the feasibility of the NIH consensus criteria to replace the old grading system of chronic GVHD (limited versus extensive) is still under evaluation. [6]
Pathophysiology
GVHD can develop in the course of (1) BMT or peripheral blood progenitor (hematopoietic stem cell) transplantation; (2) transfusion of unirradiated blood products (transfusion-associated GVHD), especially in immunocompromised individuals; or (3) solid organ transplantation involving organs containing lymphoid tissue. GVHD from passive transmission of immunocompetent maternal cells has also been described in neonates with severe immunodeficiency.
Graft-versus-host reaction occurs when donor immune cells recognize disparate host antigens. These differences are governed by genetic polymorphisms of human leukocyte antigen (HLA)-dependent factors (ie, major and minor histocompatibility antigens) and non-HLA–dependent factors (ie, cytokine gene polymorphisms, nucleotide-binding oligomerization domains [NOD2] genes, and killer immunoglobin receptor [KIR] family of natural killer [NK] receptors). [7]
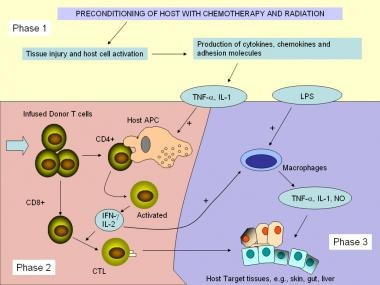
The immunopathologic characteristics of acute GVHD have often been separated into different phases (1-3), which describes the creation of a suitable host environment with the conditioning regimens intended to remove particular host cell populations, and immune-based sensitizing and efferent (effector) phases (see image below). [8, 9]
During phase 1 (Afferent phase), tissue injured by chemotherapy and irradiation releases proinflammatory cytokines such as tumor necrosis factor (TNF) alpha and interleukin (IL)-1, which subsequently increases expression of adhesion molecules, major histocompatibility complex (MHC) molecules, and costimulatory molecules. These cytokines further activate host antigen-presenting cells (APCs).
In phase 2 (Donor-T-cell activation, differentiation, and migration), the infused donor T lymphocytes are responsible for triggering GVHD and proliferate after activation by the recipient antigens expressed on host cells. APCs, such as dendritic cells or macrophages, present the antigen to CD4+ T cells, which recognize antigens in association with MHC class II molecules. [10] IL-1 produced by monocytes and other factors stimulates the T-helper cells. The T-helper cells, in turn, release compounds such as IL-2 and interferon (IFN)-γ; the latter enhances the expression of MHC class II on epithelial cells, macrophages, and dendritic cells, further stimulating the activation of T cells and NK cells.
IL-2 activates cytotoxic CD8-positive T cells, which react with MHC class I-positive targets. In addition, NK cells and macrophages appear to participate in the development of GVHD, although their roles are not well defined. Among the variables determining the extent to which GVHD develops are the types and properties of the transplanted T cells, the degree of MHC antigen mismatching, and the degree of interactions between T cells and the endothelial cells.
The final phase (3) of acute GVHD, is where immune effector cells and cytokines enact end-organ damage and contribute to a possible loss of self-tolerance. This injury is clinically manifested as the symptoms seen in GVHD and may be a contributing factor to the development of chronic GVHD.
As mentioned before, chronic GVHD develops after day 100 from transplantation and, like acute GVHD, appears dependent on alloreactivity for it to develop. Chronic GVHD has features similar to naturally occurring autoimmune disease with a wider range of involved organs. Clinical manifestations can include sclerodermatous lesions, liver failure, autoantibody production, and immune complex disease (including glomerulonephritis).
Host-recipient differences in MHC antigens or minor histocompatibility antigens can lead to this syndrome, albeit with slightly different kinetics. Alterations in thymic function with decreased thymopoiesis likely contribute to this syndrome with a breakdown of normal self-tolerance mechanisms. The donor CD4+ T-cell population is necessary for human chronic GVHD to develop; Th2 cells are the predominant subpopulation in the chronic GVHD, although the mechanisms of the disease progress remains poorly defined.
Epidemiology
Frequency
United States
Incidence and frequency of acute GVHD in transplanted or transfused populations is related to the presence of several risk factors, as follows: [11]
-
Histocompatibility: The most important factor correlating with incidence and severity of GVHD is the degree of HLA (MHC molecules in humans) disparity. With HLA-identical siblings used as bone marrow donors, the incidence of moderate-to-severe acute GVHD ranges from less than 10% to 60%, depending on prophylaxis and other risk factors (see images below). Incidence of grades II-IV acute GVHD increases to 70-75% with one HLA antigen mismatch and as much as 90% with 2-3 HLA antigen mismatch. Incidence of grades II-IV GVHD of as much as 70% have been reported in unrelated donors; a difference was noted between those receiving marrow from an HLA-identical donor or from an HLA-mismatched donor.
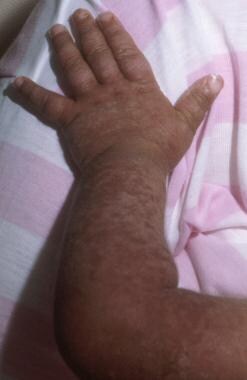 This boy developed stage III skin involvement with acute graft versus host disease (GVHD) in spite of receiving prophylaxis with cyclosporin A. The donor was an human leukocyte antigen (HLA)-matched sister; however, the sex disparity increased the risk for acute GVHD. Image courtesy of Mustafa S. Suterwala, MD.
This boy developed stage III skin involvement with acute graft versus host disease (GVHD) in spite of receiving prophylaxis with cyclosporin A. The donor was an human leukocyte antigen (HLA)-matched sister; however, the sex disparity increased the risk for acute GVHD. Image courtesy of Mustafa S. Suterwala, MD.
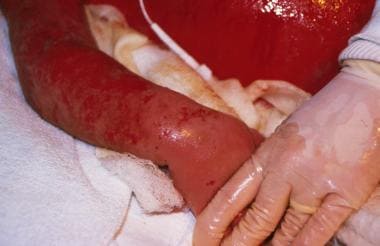 This photo depicts the same boy who has progressed to grade IV graft versus host disease (GVHD). Both cyclosporin A and methylprednisolone had been administered in high dose intravenously. He later died with chronic pulmonary disease caused by chronic GVHD. Image courtesy of Mustafa S. Suterwala, MD.
This photo depicts the same boy who has progressed to grade IV graft versus host disease (GVHD). Both cyclosporin A and methylprednisolone had been administered in high dose intravenously. He later died with chronic pulmonary disease caused by chronic GVHD. Image courtesy of Mustafa S. Suterwala, MD.
-
Graft cell composition: T-cell depletion of the bone marrow decreases the risk of GVHD but increases a risk of graft failure as well as leukemic relapse, which is due to a loss of a graft-versus-leukemia effect. Umbilical cord blood cells, when used as the source of hematopoietic stem cells, cause reduced incidence of GVHD, but the same is not true of peripheral blood stem cells.
-
Age and sex: Older patients have a significantly higher risk of acute GVHD, with an incidence of approximately 20% in the pediatric population and rising to 30% in patients aged 20-50 years and to 70% in patients aged 51-62 years. An increased risk of GVHD exists in recipients of gender-mismatched marrow, possibly because of HLA association with the Y chromosome.
-
Microenvironment: Host environment is important for the development of GVHD. Patients with aplastic anemia who are undergoing BMT and are treated with antibiotics, who are treated with skin and gut decontamination, and who are placed in a protective environment with laminar airflow units have reduced incidence of GVHD.
-
Chronic disease: Chronic GVHD develops in 30-50% of long-term survivors after BMT. [12] HLA disparity, prior acute GVHD, older age, and viral infections (especially herpesvirus group) are associated with increased risk of chronic GVHD. Chronic GVHD is also known to occur at a higher rate in survivors of transplant for aplastic anemia.
-
Type of transplantation: Acute GVHD in stem cell transplantation develops in 30-60% of recipients of sibling matched allografts. The incidence of GVHD after intestinal transplantation and liver transplantation is reported to be 5% and 0.1-1%, respectively. [13] Transfusion-associated GVHD often presents with marrow aplasia; in Japan, this is estimated to occur in 1 in 500 open-heart operations in individuals who are immunocompetent.
Mortality/Morbidity
The survival rate is 90% in grade 0-I, 60% in grade II-III, and 0 in grade IV of acute GVHD. Fatality mainly results from infections, hemorrhages, and hepatic failure. Acute GVHD can have an antileukemic effect. In chronic GVHD, the overall survival rate is 42%, with the mortality rates increased in patients with more extensive disease and thrombocytopenia.
Sex
An increased risk of GVHD is noted in recipients of sex-mismatched marrow, possibly because of HLA association with the Y chromosome.
-
Pathophysiological pathways and mechanisms of acute GVHD.
-
This boy developed stage III skin involvement with acute graft versus host disease (GVHD) in spite of receiving prophylaxis with cyclosporin A. The donor was an human leukocyte antigen (HLA)-matched sister; however, the sex disparity increased the risk for acute GVHD. Image courtesy of Mustafa S. Suterwala, MD.
-
This photo depicts the same boy who has progressed to grade IV graft versus host disease (GVHD). Both cyclosporin A and methylprednisolone had been administered in high dose intravenously. He later died with chronic pulmonary disease caused by chronic GVHD. Image courtesy of Mustafa S. Suterwala, MD.
-
Autologous graft versus host disease (GVHD) involving the skin of a patient's arm shortly after showing signs of engraftment after an autologous peripheral blood stem cell transplant for ovarian cancer. Image courtesy of Romeo A. Mandanas, MD, FACP.
-
Acute graft versus host disease (GVHD) involving desquamating skin lesions in a patient following allogeneic bone marrow transplantation for myelodysplasia. Image courtesy of Romeo A. Mandanas, MD, FACP.
-
Oral mucosal changes in a patient with chronic graft versus host disease (GVHD). Note the skin discoloration (vitiligo), which can result from GVHD. Image courtesy of Romeo A. Mandanas, MD, FACP.
-
Acute graft versus host disease (GVHD). Hematoxylin-stained and eosin-stained tissue shows dyskeratosis of individual keratinocytes and patchy vacuolization of the basement membrane. A moderate superficial dermal and perivascular lymphocytic infiltrate is also seen in this case. Image courtesy of Melanie K. Kuechler, MD.