Practice Essentials
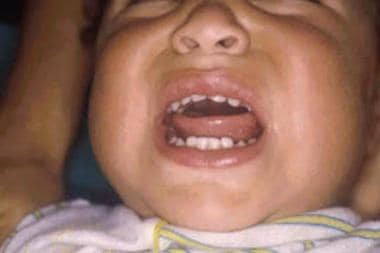
Enteroviruses are a group of single-stranded sense RNA viruses that commonly cause infections, especially in infants and children. They are responsible for a myriad of clinical syndromes including hand-foot-and-mouth (HFM) disease (see the image below), herpangina, myocarditis, aseptic meningitis, and pleurodynia.
Signs and symptoms
More than 90% of enteroviral infections are either asymptomatic or cause a nonspecific febrile illness. A wide range of symptoms is observed, but most cases include fever, a viral prodrome, and gastrointestinal symptoms.
See Presentation for more detail.
Diagnosis
The diagnosis of enteroviral infection is most often based on the clinician's assessment of the patient in conjunction with seasonal outbreaks, known exposure risks, geographic location, and age group.
Cell culture, serology, and polymerase chain reaction laboratory testing can diagnostically isolate enteroviral infections.
Other studies that may be included in the workup include the following:
-
Chest radiography
-
Echocardiography
-
Electrocardiography
-
Lumbar puncture
See Workup for more detail.
Management
No specific antiviral medication or treatment is available for an enteroviral infection. Fluid hydration and analgesia are the mainstays of care.
See Treatment for more detail.
Background
Patients with enteroviral infections may present with symptoms as benign as an uncomplicated coryzal illness or as life-threatening as encephalitis, myocarditis, or neonatal sepsis. The Centers for Disease Control and Prevention (CDC) estimate that 10-15 million cases of enteroviral infection occur each year in the United States.
Enteroviral infections result in a large number of physician and emergency department visits, creating a social and economic burden. In 1998, Pichichero et al performed a prospective study and found that nonpolio enteroviral infections resulted in direct medical costs ranging from $69 to $771 per case. [1] More recently in China, it has been estimated that each episode of enteroviral infection costs $52 to $1104 per case. [2] In addition, patients with nonpolio enteroviral infections missed a minimum of 1 day of school or camp; some missed as many as 3 days of school or camp. The significant economic and medical impacts of enteroviral infections occur mostly during the peak months of summer and fall. In temperate climates, enteroviral outbreaks occur year-round.
Enteroviruses belong to the Picornaviridae (small RNA viruses) family. The enteroviral species includes enteroviruses (EV), coxsackievirus (CV), echovirus (E), and poliovirus (PV). There are 12 species of enterovirus (A-L); however, only A to D are known to cause disease in humans. [3] The following viruses seen in humans belong to enterovirus species:
-
Enterovirus A: CVA2-8, CVA10, CVA12, CVA14, CVA16, EV-A71, EV-A76, EV-A89-92, EV-A114, EV-A119-121
-
Enterovirus B: CVB1-6, CVA9, E1-7, E9, E11-21, E24-27, E29-33, EVB69, EV-B73-75, EV-B77-88, EV-B93, EV-B97-98, EV-B100-101, EV-B106-107, EV-B111
-
Enterovirus C: PV1-3, CVA1, CVA11, CVA13, CVA17, CVA19-22, CVA24, EV-C95-96, EV-C99, EV-C102, EV-C104-105, EV-C109, EV-C113, EV-C116-118
-
Enterovirus D: EV-D68, EV-D70, EV-D94, EV-D111
More recently, a related genus of viruses, Parechovirus, has been described; two enterovirus species (echovirus types 22 and 23) were reassigned as parechovirus. [4]
Clinically, enteroviruses can be divided into polio and non-polio enteroviruses owing to the different clinical patterns and outcomes. Polioviruses can cause poliomyelitis, which causes muscle paralysis. Non-polio enteroviruses commonly cause illness such as HFM, herpangina, and conjuncitivitis but can also cause more burdensome conditions, such as aseptic meningitis and myocarditis. There is currently no established treatment for enteroviruses; however, drugs and vaccines are undergoing clinical research at present.
Enterovirus A71 has gained notoriety in recent years for causing a rapidly fatal rhombencephalitis, in association with epidemics of HFM disease in East Asian countries. This appears to be a particularly aggressive neurotrophic serotype of enterovirus.
Coxsackievirus A6 was recently described as a somewhat distinct clinical entity of "atypical hand foot mouth disease," as the skin lesions described are vesiculobullous rather than the typical flat ulcers seen in HFM disease and may be more extensive, often involving areas of preexisting eczema.
Each virus obtains its antigenicity from the capsid proteins that surround the RNA core. According to the CDC, 65 human serotypes of enteroviruses have been identified; however, a small number cause most outbreaks. Since the implementation of polio vaccines, the incidence of wild-type polio has been almost eradicated.
The most common form of human transmission is the fecal-to-oral route. Although respiratory and oral-to-oral routes are possible, they are more likely to occur in crowded living conditions. Enteroviruses are quite resilient. They remain viable at room temperature for several days and can survive the acidic pH of the human gastrointestinal (GI) tract. The incubation period is usually 3-10 days.
Pathophysiology
The enterovirus enters the human host through the GI or respiratory tract. The cell surfaces of the GI tract serve as viral receptors, and initial replication begins in the local lymphatic GI tissue. The virus seeds into the bloodstream, causing a minor viremia on the third day of infection. The virus then invades organ systems, causing a second viremic episode on days 3-7. This second viremic episode is consistent with the biphasic prodromal illness. The infection can progress to central nervous system (CNS) involvement during the major viremic phase or at a later time. Antibody production in response to enteroviral infections occurs within the first 7-10 days.
Coxsackievirus replicates in the pharynx (herpangina), the skin (HFM disease), the myocardium (myocarditis), and the meninges (aseptic meningitis). It can also involve the adrenal glands, pancreas, liver, pleura, and lung.
Echovirus can replicate in the liver (hepatic necrosis), the myocardium, the skin (viral exanthems), the meninges (aseptic meningitis), the lungs, and the adrenal glands.
After exposure, poliovirus replicates in the oropharynx and GI tissue. Following this replication, poliovirus advances, invading the motor neurons of the anterior horn cells of the spinal cord. It can progress to other CNS regions, including the motor cortex, cerebellum, thalamus, hypothalamus, midbrain, and medulla, causing the death of neurons and paralysis. Neuropathy occurs as a result of direct cellular destruction. Antibody production occurs in the lymphatic system of the GI tract, prior to invasion of the CNS tissue. Infants retain transplacental immunity for the first 4-6 months of life.
The enteroviruses are capable of directing almost all cellular protein translation to viral genes through the modification of host cell translation factors (messenger RNA [mRNA] cap-binding proteins) and using internal ribosome entry sites (IRES) to bypass the crippled host machinery. As such, they are highly damaging to the cells they infect.
Etiology
Enteroviral risk factors include poor sanitation, crowded living conditions, and lower socioeconomic class status. In addition, children younger than 5 years are more susceptible because of poor hygiene habits and lack of prior immunity.
Although debatable, neonatal infections are most likely acquired after birth rather than transplacentally. Exposure from an infected mother or another infant in the nursery during the first 2 weeks of life is the probable mode of transmission. The enteroviral exposure may be perineally acquired during the delivery process.
A B-cell response is needed for the host to properly fight off the enteroviral infection and to prevent entry to the CNS. Children who lack a functioning B-cell system, such as those with X-linked agammaglobulinemia, are at risk of serious enteroviral infection, such as meningoencephalitis.
Poliovirus is a consideration in all unimmunized or partially immunized children.
Epidemiology
United States data
Nonpolio
Nonpolio enteroviral infections cause an estimated 10-15 million symptomatic infections per year in the United States. Many are treated as potential episodes of sepsis, and antibiotics and acyclovir are administered to treat possible bacterial or herpetic infection. Children under 5 years of age are predominantly affected. [5] Outbreaks tend to occur in warmer months.
The CDC reported a 2014 outbreak of enterovirus D68 that began in at least six US states from mid-August to mid-September, including Colorado, Illinois, Iowa, Kansas, Kentucky, and Missouri. [6] This outbreak caused 1153 cases of severe respiratory disease and 107 cases of acute flaccid myelitis (AFM) in the United States alone. [7] Outbreaks spread across the United States, then Canada, Europe, and Asia. Enterovirus D68 was first identified in 1962 in California but had not been commonly reported in the United States before the 2014 outbreak. Children with underlying immunodeficiency and respiratory disease were noted to have a higher risk of severe respiratory illness from enterovirus D68. [7]
A 2014 outbreak in California of echovirus 30 caused 10 cases of viral meningitis; the outbreak was centered around the junior varsity American football team. [8] In 2018, an outbreak of enterovirus A71 causing CNS disease was reported in Colorado. This outbreak caused meningitis, encephalitis, and AFM. [5] There were no deaths, but 3 children with AFM had residual limb weakness at discharge.
Polio
In 1952, an epidemic of polio occurred in the United States, causing 3,000 deaths and 57,879 cases. The vaccine has virtually eliminated wild-type polio in the United States. In 1994, the World Health Organization (WHO) declared the eradication of wild polio in the Western hemisphere; however, 8-10 cases of vaccine-associated paralytic polio (VAPP) continued to occur annually in the US from 1980 to 1999. [9] Such cases occur when the attenuated vaccine virus in the oral polio vaccine (OPV) is excreted into an under-immunized population and undergoes genetic changes that cause it to become virulent again. VAPP is linked to the concomitant administration of live OPV with intramuscular injections (perhaps allowing the virus better access to myocytes and neuronal axons) and occurs in 1 per 2-4 million vaccinations, whereas paralytic polio occurs in 1 in 200 wild-type infections. In 2000, the risk of VAPP became greater than wild type disease, leading to the change from live OPV to the inactivated polio vaccine (IPV).
In 1979, an outbreak occurred in numerous Amish communities throughout the United States. A smaller outbreak occurred in 2005 in an Amish community in Minnesota. Genetic sequencing of the virus surprisingly revealed that it was only 2.3% different from the Sabin OPV strain and was likely acquired from overseas subclinical circulating infections.
International data
Nonpolio
Nonpolio enteroviral infections are prevalent worldwide, with clear outbreaks of HFM, herpangina, and aseptic meningitis occurring as well as non-specific viral illneses. [10, 11, 12]
Between 1999 and 2007, the Austrian reference laboratory for poliomyelitis received 1,388 stool specimens for enterovirus typing from patients with acute flaccid paralysis or aseptic meningitis; 201 samples from 181 cases were positive for nonpolio enterovirus. [13] The mean patient age was 5-6 years, with 90% of cases in children younger than 14 years. Aseptic meningitis was identified in 65.6% of the cases. Echovirus 30 was the most frequent viral cause of aseptic meningitis, followed by coxsackievirus B types 1-6 and enterovirus 71. E-30 was also the leading viral pathogen in a Spanish study of aseptic meningitis. [14]
An outbreak of echovirus 30 infection occurred between April and September 2013 in Marseille, France. A study concluded that almost all E-30 infections emerged from local circulation of one parental virus. The findings also showed that human enterovirus outbreaks cause excess of emergency ward consultations and consultations to general practitioners for non-specific viral illness. [15] Similar outbreaks have been reported in Germany, Finland, Italy, and China. [16, 17, 18, 19]
Polio
Poliomyelitis still occurs in low-income countries as a result of poor health care and an inability to access vaccines. However, the reduction in global cases of polio is a testament to the effectiveness of vaccination programs and the WHO strategy to eradicate polio globally. [20] The WHO reported over 35,000 cases globally in 1988, then only 33 in 2018. [21] This significant drop is due to aggressive vaccination programs following a resolution at the World Health Assembly in 1988 to eradicate polio. The WHO estimates that the vaccination program has saved 16 million people from paralysis.
In September 2019 the WHO announced that poliovirus 2 and 3 have now been eradicated globally. [22] Poliovirus 1 remains endemic in Afghanistan and Pakistan, but no new cases have been detected in Africa since 2016. [21] In 2018, 33 cases of poliovirus 1 were detected in Afghanistan and Pakistan, where there is incomplete vaccination, in part due to political and social instability. [21]
Some genetic evidence suggests that if the poliovirus is eradicated, genetic recombination between other enteroviruses may result in a phenotypically similar virus. However, this appears to be of academic interest only at this time.
Race-, sex-, and age-related demographics
Enteroviruses have a worldwide distribution and are not race-specific infections.
Males and females are equally affected. Males are more likely to be symptomatic.
People of all ages, including adults, elderly people, and young people, are at risk of manifesting symptoms of enteroviruses. Children have a higher rate of infection because of exposure, hygiene, and immunity status. [23] Unlike most cases of nonpolio enteroviral infections, acute hemorrhagic conjunctivitis occurs most frequently in adults aged 20-50 years.
Prognosis
The prognosis for nonpolio enteroviral infections is excellent. Adverse outcomes are specifically related to cases of newborn infections and older children with myocarditis and encephalitis.
In most cases of polio, patients have some return of muscle function. Prognosis of final ability is determined 6 months or longer following the infection.
Morbidity/mortality
The overall mortality rate for nonpolio enteroviruses is extremely low. The patients at greatest risk are those with neonatal sepsis or myocarditis or those with underlying immunodeficiency and respiratory disease. [7] One retrospective review of 30 hospitalized children with enterovirus infections in southern England described a mortality rate of 10%. [23]
Occasionally, enteroviruses cause global encephalitis, which has a good prognosis. However a few fatalities have been reported. Enterovirus 71 has been linked with a rhombencephalitis (inflammation of the brain stem) in outbreaks of hand-foot-and-mouth disease in the eastern hemisphere (Taiwan, Japan, Malaysia, and Australia). Fatality rates from these outbreaks have been as high as 14%. Myoclonus is a poor prognostic indicator, as are lethargy, persistent fever, and peak temperature higher than 101.3ºF (38.5ºC). [24]
The 2014 global outbreak of enterovirus D68 caused 14 deaths in the United States from a total of 1152 hospitalizations. [7] Most cases of myocarditis and pericarditis are self-limited, but a potentially significant mortality rate is associated with myocarditis and some children have persistent myocardial dysfunction. [23] Older patients can develop a dilated cardiomyopathy following myocarditis.
The overall mortality rate for paralytic polio is 5-10%; death usually results from paralysis of the respiratory muscles. [25] For those who survive, a 6-month period is allowed to predict how much muscle function will return.
Complications
Both coxsackievirus and enterovirus have been associated with the development of Guillain-Barré syndrome.
Enteroviruses, specifically coxsackie B, have been hypothesized to play a role in the development of type 1 diabetes. This is based on epidemiological studies and that in post-mortem and in vitro studies have suggested that coxsackie B has a predilection for the pancreatic islet cells. Rodent models have demonstrated enteroviral destruction of pancreatic beta-islet cells, but no definitive link has been established. [26]
Several studies have investigated a possible link between enteroviral infections and increased risk of myocardial infarction, but no definite conclusions have been proven.
-
Erosions on the base of the tongue.
-
A red halo surrounds several vesicles on the finger flexures and palms.
-
Small linear vesicle on the thumb.
-
Vesicle on the dorsal hand of a young adult.
-
Calf blisters from coxsackievirus A6 as seen in atypical hand-foot-mouth disease. Courtesy of Elsevier (Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. Jan 2014;14(1):83-6).