Practice Essentials
Bronchopulmonary dysplasia (see the image below) is a form of chronic lung disease that develops in preterm neonates treated with oxygen and positive-pressure ventilation. The pathogenesis of this condition remains complex and poorly understood; however various factors can not only injure small airways but also interfere with alveolarization (alveolar septation), leading to alveolar simplification with a reduction in the overall surface area for gas exchange. The developing pulmonary microvasculature can also be injured.
Signs and symptoms
Many infants born with bronchopulmonary dysplasia exhibit signs and symptoms of respiratory distress syndrome, including the following:
-
Tachypnea
-
Tachycardia
-
Increased respiratory effort (with retractions, nasal flaring, and grunting)
-
Frequent desaturations
These infants are often extremely immature, have a very low birth weight, and have significant weight loss during the first 10 days of life. Their requirements for oxygen and ventilatory support often increase in the first 2 weeks of life. At weeks 2-4, oxygen supplementation, ventilator support, or both are often increased to maintain adequate ventilation and oxygenation.
See Presentation for more detail.
Diagnosis
Laboratory tests
Laboratory studies used to evaluate and monitor infants with bronchopulmonary dysplasia include the following:
-
Arterial blood gas (ABG) levels: To assess for acidosis, hypercarbia, and hypoxia (with increased oxygen requirements)
-
Transcutaneous or end-tidal carbon dioxide levels: To evaluate trends, especially if the results are correlated with ABG levels
-
Pulmonary function tests
Continuously monitor oxygenation by using pulse oximetry because of frequent desaturations. In addition, routinely monitor blood pressure, as infants with bronchopulmonary dysplasia can also develop systemic hypertension.
Imaging studies
The following radiologic studies may be used to evaluate infants with suspected bronchopulmonary dysplasia:
-
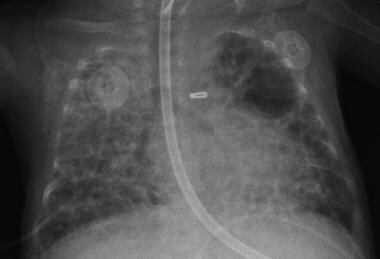
Chest radiography: To determine the severity of bronchopulmonary dysplasia; to differentiate bronchopulmonary dysplasia from atelectasis, pneumonia, and air leak syndrome; to demonstrate decreased lung volumes, areas of atelectasis and hyperinflation, pulmonary edema, and pulmonary interstitial emphysema
-
High-resolution chest computed tomography scanning
-
Chest magnetic resonance imaging
Procedures
Biopsy of the lungs in preterm infants with bronchopulmonary dysplasia may reveal findings from the following 4 pathologic stages:
-
Acute lung injury
-
Exudative bronchiolitis
-
Proliferative bronchiolitis
-
Obliterative fibroproliferative bronchiolitis
See Workup for more detail.
Management
In most cases of bronchopulmonary dysplasia, respiratory distress syndrome is diagnosed and treated using the following:
-
Surfactant replacement with oxygen supplementation
-
Continuous positive airway pressure (CPAP)
-
Mechanical ventilation
Prenatal management in the pregnant mother to lower the risk bronchopulmonary dysplasia in the infant includes the following:
-
Treatment of the maternal inflammatory conditions, such as chorioamnionitis [1]
-
Treatment of maternal infection, such as Ureaplasma urealyticum infection [2]
Diet
Infants with bronchopulmonary dysplasia have increased energy requirements. The following nutritional strategies may help infants and their lungs grow and develop:
-
Administration of early parenteral nutrition
-
Maximization of protein, carbohydrates, fat, vitamins, and trace metals intake
-
Supplementation with antioxidant enzymes and vitamins A and E
-
Administration of free water (avoid fluid overload)
-
Protein and fat supplementation
-
Early enteral feeding of small amounts (even with umbilical lines in place), followed by slow, steady increases in volume: To optimize tolerance of feeds and nutritional support
Pharmacotherapy
The following medications are used in the management of bronchopulmonary dysplasia:
-
Diuretics (eg, furosemide)
-
Bronchodilators (eg, albuterol, caffeine citrate, theophylline, ipratropium bromide)
-
Corticosteroids (eg, dexamethasone)
-
Vitamins (eg, vitamin A)
See Treatment and Medication for more detail.
Background
Bronchopulmonary dysplasia (BPD) is a form of chronic lung disease that develops in preterm neonates treated with oxygen and positive-pressure ventilation (PPV).
Northway et al reported clinical, radiographic, and histologic changes in the lungs of preterm infants who had respiratory distress syndrome (RDS) and were treated with oxygen and mechanical ventilation. [3]
Northway et al's original definition has been extensively modified over the last 4 decades. Bancalari et al’s definition involves ventilation criteria, an oxygen requirement at 28 days to maintain arterial oxygen tensions of more than 50 mm Hg, and abnormal findings on chest radiography. [4] Shennan et al proposed that an additional need for supplemental oxygenation at 36 weeks' postmenstrual age may be the most accurate indicator of pulmonary outcome; [5] this criterion decreased the large number of relatively healthy preterm infants Bancalari and others included in their definitions.
Jobe and Bancalari summarized proceedings of a National Institute of Health consensus conference on bronchopulmonary dysplasia. [6] Investigators from the National Institute of Child Health and Human Development (NICHD) have validated their recommendations. This group improved the definition of bronchopulmonary dysplasia and attempted to assign a severity score based on oxygen requirements and the need for respiratory support. However, physicians and institutions may set different standards for oxygen requirements and for target ranges for oxygen saturation. This variation in practice may notably influence the incidence and severity of bronchopulmonary dysplasia in a particular neonatal ICU (NICU).
To overcome this limitation due to subjectivity in "need for oxygen," Walsh et al recently developed a physiologic definition of bronchopulmonary dysplasia. [7] According to this definition, at 35-37 weeks' postmenstrual age, infants treated with mechanical ventilation, continuous positive airway pressure (CPAP), or supplemental oxygen concentration of 30% and oxygen saturations of 90-96% were diagnosed with bronchopulmonary dysplasia without additional testing. Infants with supplemental oxygen concentrations of 30% at rest with oxygen saturations of 90-96% or supplemental oxygen concentrations of 30% with oxygen saturations of more than 96% underwent a timed stepwise reduction to room air.
For infants receiving oxygen by hood, oxygen was weaned in 2% increments. For infants receiving oxygen by nasal cannulae, flow was initially weaned in increments (for flow of 1–2, step down 0.5 liters per minute [lpm]; for flow 0.1-0.99 lpm, step down 0.1 lpm), and then the oxygen concentration was reduced in increments of 20% to room air. Cannulae were removed from the nares for the remainder of the challenge. Oxygen that was given only during feedings was not included for the purposes of eligibility. Those who failed the reduction were diagnosed with bronchopulmonary dysplasia.
No bronchopulmonary dysplasia was defined by requiring treatment with room air with oxygen saturation of more than 90% or passing a timed, continuously monitored oxygen-reduction test. [7] The physiologic definition of bronchopulmonary dysplasia reduced the overall rate of bronchopulmonary dysplasia and reduced the variation among centers. The physiologic definition may facilitate the measurement of bronchopulmonary dysplasia as an outcome in clinical trials and the comparison between and within centers over time.
Recently, models have been developed for predicting the probability of BPD at specific postnatal time points using readily available clinical data. [8] Prediction improved with advancing postnatal age, increasing from a C statistic (area under the curve) of 0.79 on day 1 to a maximum of 0.85 on day 28. On postnatal days 1 and 3, gestational age best improved outcome prediction, while type of respiratory support was most important on postnatal days 7, 14, 21, and 28. [8]
Pathophysiology
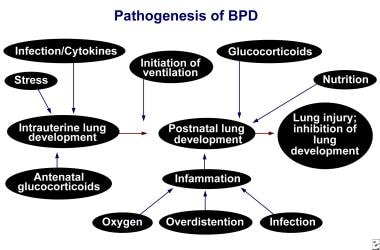
The pathogenesis of bronchopulmonary dysplasia remains complex and poorly understood. Bronchopulmonary dysplasia results from various factors that can injure small airways and that can interfere with alveolarization (alveolar septation), leading to alveolar simplification with a reduction in the overall surface area for gas exchange. The developing pulmonary microvasculature can also be injured. Alveolar and lung vascular development are intimately related, and injury to one may impair development of the other. Damage to the lung during a critical stage of lung growth can result in clinically significant pulmonary dysfunction.
Premature birth and subsequent events (eg, exposure to oxygen, mechanical ventilation, inflammatory agents, infection) likely shifts the balance from lung development consisting of lung alveolar and vascular growth to one of premature maturation, which is associated with an arrest in development and a loss of future gas exchange area; however, alveolar maturation might facilitate gas exchange in the short-term. [9]
While inflammation is associated with development of BPD, the role of chorioamnionitis in development of BPD after adjustment for prematurity is uncertain. Recent large studies indicate that chorioamnionitis is not associated with BPD. [10, 11] However, alterations in the airway microbiome at birth have been noted in infants exposed to chorioamnionitis, and these alterations have been found to be associated with BPD. [12]
Epidemiology
United States data
Infants with severe bronchopulmonary dysplasia are often extremely immature and have very low birth weight, although term infants with severe respiratory failure are also at increased risk. Bronchopulmonary dysplasia is uncommon in infants with a birth weight of more than 1250 g and in infants who were born at more than 30 weeks' gestation. Overall, about one fourth of infants who weigh less than 1500 g are diagnosed with bronchopulmonary dysplasia.
Antenatal glucocorticosteroids, early surfactant therapy, and gentle modalities of ventilation have minimized the severity of lung injury, particularly in relatively mature infants. However, improved survival has increased the prevalence of bronchopulmonary dysplasia, especially in small infants who may have been exposed to in utero infection (eg, chorioamnionitis).
Several trials of surfactants revealed that incidences of bronchopulmonary dysplasia widely vary, from 17-57%. No substantial difference between placebo-treated and surfactant-treated survivors has been reported. Kresch and Clive performed a meta-analysis of surfactant-replacement therapy for infants weighing less than 2 kg. [13] Infants receiving modified natural surfactant had improved survival without bronchopulmonary dysplasia. Van Marter and associates described the wide variation in the prevalence of bronchopulmonary dysplasia in different NICUs using various ventilatory strategies. [14] This variation has also been noted among sites in the Vermont Oxford Network (VON) and in the NICHD research network, suggesting that differences in patient populations and clinical practices may directly affect outcomes.
International data
Studies similar to those in the United States have been conducted to compare rates of bronchopulmonary dysplasia in different NICUs in Europe. Results have been similar despite the relatively homogeneous population.
Race-, sex-, and age-related demographics
Compared with white infants, African American infants generally have a lower incidence of severe bronchopulmonary dysplasia, although the combined rate of bronchopulmonary dysplasia and death is often similar in persons of different races. [8]
Male infants with bronchopulmonary dysplasia tend to have more severe disease and worse neurodevelopmental outcome.
Bronchopulmonary dysplasia is most common in the most immature neonates born at 22-30 weeks' gestational age. These patients frequently weigh less than 1000 g at birth.
Prognosis
Prognosis
Most neonates with bronchopulmonary dysplasia ultimately survive. Note the following:
-
As infants, patients are at increased risk for repeated and serious pulmonary infections (eg, respiratory syncytial virus [RSV]), asthma, cardiac dysfunction, and neurologic impairments.
-
Infants with severe bronchopulmonary dysplasia remain at high risk for pulmonary morbidity and mortality during the first 2 years of life.
-
Rehospitalization for impaired pulmonary function is most common during the first 2 years of life.
-
Hakulinen and associates found a gradual decrease in symptom frequency among children aged 6-9 years compared with infants aged 0-2 years. [15]
-
In children and adults with a history of bronchopulmonary dysplasia, high-resolution chest CT reveals lung abnormalities that are directly correlated with the degree of pulmonary dysfunction.
-
The infant with severe bronchopulmonary dysplasia is at high risk for long-term pulmonary and neurologic sequelae.
-
Persistent right ventricular hypertrophy or fixed pulmonary hypertension unresponsive to oxygen supplementation is associated with a poor prognosis.
-
Northway followed up pediatric patients with bronchopulmonary dysplasia to adulthood and reported that patients had airway hyperreactivity, abnormal pulmonary function, and hyperinflation, as noted on chest radiography. [16]
Morbidity/mortality
Postnatal infection and/or sepsis, periventricular leukomalacia (PVL), severe intraventricular hemorrhage, ventriculomegaly, hearing impairment, and severe retinopathy of prematurity (ROP) are all important confounding variables that can greatly affect an infant's outcome.
Since the introduction of surfactant replacement, survival of the most immature infants has improved. However, the stable 25-50% survival rates in preterm infants at 23-24 weeks' gestation likely reflect the lack of alveolarization and vascular development. Survival and morbidity improved in infants older than 24 weeks' gestation after the widespread administration of antenatal corticosteroids was introduced in 1994.
Along with other advances in technology and an improved understanding of neonatal physiology, infants with bronchopulmonary dysplasia appear to have milder disease today than in years past.
Infants with severe bronchopulmonary dysplasia remain at high risk for pulmonary morbidity and mortality during the first 2 years of life. Infants with bronchopulmonary dysplasia are at risk for repeated pulmonary infections and asthma requiring repeated hospital admissions and office visits.
Abnormal long-term neurodevelopmental outcome, muscular development, slow growth, and chronic pulmonary morbidity are common in infants with bronchopulmonary dysplasia. Whether abnormal neurodevelopmental outcomes are directly related to bronchopulmonary dysplasia or to the patients' marked immaturity and disease severity is hard to determine.
-
Prenatal Influences on the development of bronchopulmonary dysplasia (BPD).
-
Bronchopulmonary dysplasia (BPD).
-
Chest radiograph of infant with bronchopulmonary dysplasia.