Practice Essentials
Both community-associated and hospital-acquired infections with Staphylococcus aureus have increased in the past 20 years, and the rise in incidence has been accompanied by a rise in antibiotic-resistant strains—in particular, methicillin-resistant S aureus (MRSA) and, more recently, vancomycin-resistant strains.
An example of radiographic findings in S aureus infections is shown in the image below.
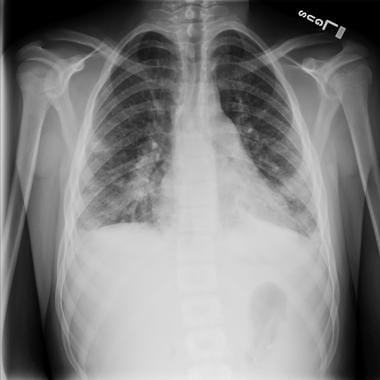 Posteroanterior chest radiograph of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing borderline cardiomegaly, multiple nodular infiltrates, and bilateral pleural effusions.
Posteroanterior chest radiograph of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing borderline cardiomegaly, multiple nodular infiltrates, and bilateral pleural effusions.
Signs and symptoms
Types and presentation of S aureus infection include the following:
-
Skin and soft tissue (impetigo): A small area of erythema that progresses into bullae (filled with cloudy fluid) that rupture and heal with the formation of a honey-colored crust
-
Scalded skin syndrome (Ritter disease): A relatively rare, toxin-mediated disorder with superficial fragile blisters that burst, leaving a tender base; often accompanied by fever and occasionally by mucopurulent eye discharge
-
Folliculitis: A tender pustule that involves the hair follicle
-
Furuncle: Small abscesses characterized by exuding purulent material from a single opening; involves both the skin and the subcutaneous tissues in areas with hair follicles
-
Carbuncle: An aggregate of connected furuncles, with several pustular openings
-
Bone infections (osteomyelitis): In children, sudden onset of fever and bony tenderness or a limp; pain may be throbbing and severe; however, presentation in neonates can be subtle
-
Septic arthritis: Decreased range of motion, warmth, erythema, and tenderness of the joint with constitutional symptoms and fever; however, these signs may be absent in infants (in whom the hip is the most commonly involved joint)
-
Endocarditis: Initially presents as fever and malaise; peripheral emboli may be present; may involve healthy valves
-
Toxic shock syndrome: Fever, diffuse macular erythema, and hypotension, with involvement of 3 or more organ systems; can be rapidly progressive in previously healthy individuals
-
Pneumonia: Most common in infants, young children, and debilitated patients; a short prodrome of fever followed by rapid onset of respiratory distress; prominent GI symptoms may also occur
-
Thrombophlebitis: Fever, pain, and occasionally erythema at the insertion site of an intravenous catheter; usually affects hospitalized patients
See Clinical Presentation for more detail.
Diagnosis
Folliculitis, furuncle, and carbuncle
-
Diagnosis based on clinical appearance
-
Aspiration or incision and culture of purulent material from the lesion occasionally diagnostic
Osteomyelitis
-
Cultures of bone aspirate
-
Blood culture results positive in only 30-50% of pediatric patients
-
C-reactive protein levels and erythrocyte sedimentation rate are generally elevated in acute disease
-
Bone scan with increased technetium-99m–labeled diphosphonate uptake supports the clinical diagnosis; however, this modality is not as useful in neonates or after trauma or surgery
-
MRI is the best imaging modality for defining purulent collections and for planning surgery
-
On plain film radiographs, destructive bone changes are usually observed 2 weeks after infection
Septic arthritis
-
Gram stain and culture of joint fluid is the primary means of diagnosis
-
Direct inoculation of synovial fluid into culture bottles may improve culture yield
-
Median white blood cell count in joint fluid is 60.5 × 109, with neutrophil predominance >75%
-
Synovial fluid glucose levels are often low
-
Plain radiographs show capsular swelling
-
MRI or CT scanning is the imaging method of choice for pyogenic sacroiliitis
Endocarditis
-
Blood culture is the most important diagnostic procedure
-
Inject the blood sample into hypertonic media if the patient has been exposed to antibiotics
-
Obtain 3-5 sets of large-volume blood cultures within the first 24 hours
-
Echocardiography is a valuable adjunct
Pneumonia
-
Blood cultures are more likely to be positive in secondary than primary disease (90% vs 20%)
-
An adequate respiratory tract specimen should be obtained prior to initiating therapy; specimens may include endotracheal sampling, pleural fluid, or lung tap
-
Sputum specimens are inadequate because upper respiratory tract colonization is common
-
No radiologic features are highly specific
-
Typical radiographic features are unilateral consolidation in primary staphylococcal pneumonia and bilateral infiltrates in secondary cases
-
Early in the disease course, the chest radiograph may reveal minimal infiltrates, but within hours, infiltrates progress rapidly
-
Pleural effusion, pneumatoceles, and pneumothorax are also common
-
In oncology patients, S aureus may cause pulmonary nodules [7]
Thrombophlebitis
-
Obtain a blood culture through the intravenous line and a peripheral blood culture
See Workup for more detail.
Management
Antibiotic regimens include the following:
-
Empiric therapy with penicillins or cephalosporins may be inadequate because of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) [8]
-
Combination therapy with a penicillinase-resistant penicillin or cephalosporin (in case the organism is methicillin-sensitive S aureus [MSSA]) [9] and clindamycin or a quinolone
-
Clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), rifampin, doxycycline, or a quinolone
-
TMP-SMX and rifampin in combination, rather than singly
-
Clindamycin (rather than TMP-SMX) may become the preferred outpatient antibiotic therapy in regions with a relatively low incidence of clindamycin resistance [10]
-
The Infectious Diseases Society of America has published treatment guidelines for MRSA infection [11]
Treatment of specific infections
Impetigo, folliculitis, furuncle, carbuncle:
-
Superficial or localized skin infections: A topical agent such as mupirocin or retapamulin; however, most CA-MRSA strains are (or readily become) resistant to mupirocin
-
More extensive or serious skin disease and bullous impetigo: Oral antistaphylococcal agents [12]
-
Drainage of pus collections is of paramount importance [13]
Scalded skin syndrome (Ritter disease):
-
Eradication of the focal infection to end toxin production
-
Large doses of intravenous antistaphylococcal agents such as oxacillin or a first-generation cephalosporin such as cefazolin, combined with clindamycin
Osteomyelitis:
-
Empiric semisynthetic penicillin and clindamycin
-
In patients with allergy to penicillin, a first-generation cephalosporin and clindamycin
-
Vancomycin or linezolid when the other drugs mentioned are absolutely not tolerated or when resistance or the clinical course dictates
-
Minimum effective treatment time is 4-6 weeks; therapy can be completed orally [14]
-
Surgery to drain purulent material from the subperiosteal space or remove infected foreign material
Septic arthritis:
-
A parenteral antistaphylococcal drug (eg, oxacillin, which is penicillinase resistant; clindamycin; cefazolin)
-
Therapy usually continues for at least 4 weeks; duration of parenteral therapy is debated
-
Joint fluid that reaccumulates should be removed and a sample should be cultured
-
Hip or shoulder infections in infants require prompt drainage to prevent bony destruction
-
Surgical drainage is indicated if needle drainage is inadequate
Endocarditis:
-
The combination of a beta-lactam and an aminoglycoside (eg, nafcillin and gentamicin)
-
In patients with MRSA, combinations of vancomycin with aminoglycosides
-
Rifampin can be added to combination therapy, especially for prosthetic valve endocarditis
-
Duration of therapy is at least 4 weeks
-
Bacteremia, fever, and leukocytosis for at least a week after therapy is initiated
Toxic shock syndrome:
-
Surgical exploration and drainage of all potential foci of infection
Thrombophlebitis:
-
Removal of the infected intravenous line in patients who are immunocompromised or severely ill or when infection cannot be eradicated medically
Bacteremia :
-
Daptomycin, with or without beta-lactams, controls S aureus bacteremia without worsening renal dysfunction. In a cohort of patients with mild or moderate renal insufficiency, more than 80% responded to treatment, with no detrimental effect on their kidneys. Currently, the combination of daptomycin with beta-lactams is recommended only as salvage therapy for refractory MRSA bacteremia. [15]
See Treatment and Medication for more detail.
Background
Bacteria of the genus Staphylococcus are gram-positive cocci that are microscopically observed as individual organisms, in pairs, and in irregular, grapelike clusters. The term Staphylococcus is derived from the Greek term staphyle, meaning "a bunch of grapes." Staphylococci are nonmotile, non–spore-forming, and catalase-positive bacteria. The cell wall contains peptidoglycan and teichoic acid. The organisms are resistant to temperatures as high as 50°C, to high salt concentrations, and to drying. Colonies are usually large (6-8 mm in diameter), smooth, and translucent. The colonies of most strains are pigmented, ranging from cream-yellow to orange.
The ability to clot plasma continues to be the most widely used and generally accepted criterion for the identification of Staphylococcus aureus. One such factor, bound coagulase, also known as clumping factor, reacts with fibrinogen to cause organisms to aggregate. Another factor, extracellular staphylocoagulase, reacts with prothrombin to form staphylothrombin, which can convert fibrinogen to fibrin. Approximately 97% of human S aureus isolates possess both of these forms of coagulase.
S aureus is ubiquitous and may be a part of human flora found in the axillae, the inguinal and perineal areas, and the anterior nares. von Eiff et al described 3 patterns of carriage: those who always carry a strain, those who carry the organism intermittently with changing strains, and a minority of people who never carry S aureus. [16] Persistent carriage is more common in children than in adults. Nasal carriers may be divided into persistent carriers with high risk of infection and intermittent or noncarriers with low risk of infection. [17]
Wenzel and Perl found that, among healthy adults, carrier rates of 11-32% were detected in the general population, and a prevalence of 25% was detected in hospital personnel. [18] Using pulsed-field gel electrophoresis (PFGE) for molecular typing, von Eiff et al found that, in most patients with S aureus bacteremia, the isolate from the patient's blood is identical to that found in the anterior nares. [16] Persistent nasal carriage depends on host genetic determinants. [19]
Curiously, community-associated methicillin-resistant S aureus (CA-MRSA) is less often found in the anterior nares than are methicillin-sensitive S aureus (MSSA) and hospital-acquired methicillin-resistant S aureus (HA-MRSA) [20, 21, 22, 23] ; rather, it colonizes the skin, particularly in the perineal area [24] and the rectum. [25] It also colonizes the pharynx, [26] gut, [27] and vagina. [28, 29] Another interesting finding is the protective effect of consumption of hot coffee or tea for nasal carriage of MRSA. [30] In contrast, hormonal contraceptive use is a significant risk factor for nasal carriage of S aureus. [31] [#IntroductionPathophysiology]
Pathophysiology
The organism may cause disease through tissue invasion and toxin production. [32] The toxins liberated by the organism may have effects at sites distant from the focus of infection or colonization.
Tissue invasion
The postulated sequence of events that leads to infection is initiated with carriage of the organism. The organism is then disseminated via hand carriage to body sites where infection may occur (either through overt breaks in dermal surfaces, such as vascular catheterization or operative incisions, or through less evident breakdown in barrier function, such as eczema or shaving-associated microtrauma).
The hallmark of staphylococcal infection is the abscess, which consists of a fibrin wall surrounded by inflamed tissues enclosing a central core of pus containing organisms and leukocytes. From this focus of infection, the organisms may be disseminated hematogenously, even from the smallest abscess. The ability to elaborate proteolytic enzymes facilitates the process. This may result in pneumonia, bone and joint infection, and infection of the heart valves. In immunocompromised hosts (eg, patients with cancer who are neutropenic and have a central venous line), 20-30% develop serious complications or fatal sepsis following catheter-related S aureus bacteremia.
Persistent deep-seated infections have now been linked to small-colony variants of the organism. [33] This population is more resistant to antibiotics and grows slowly. These organisms have been described in patients with cystic fibrosis and may contribute to the persistence of S aureus in these patients.
Toxin-mediated disease
The organism also elaborates toxins that can cause specific diseases or syndromes and likely participate in the pathogenesis of staphylococcal infection. [34] Enterotoxin-producing strains of S aureus cause one of the most common food-borne illnesses. The most common presentation is acute onset of vomiting and watery diarrhea 2-6 hours after ingestion. The symptoms are usually self-limited. The cause is the proliferation of toxin-producing organisms in uncooked or partially cooked food that an individual carrying the staphylococci has contaminated.
A rare but well-described disorder in neonates and young children is staphylococcal scalded skin syndrome (Ritter disease). The organism produces an exfoliative toxin produced by strains belonging to phage group II. Initial features include fever, erythema, and blisters, which eventually rupture and leave a red base. Gentle shearing forces on intact skin cause the upper epidermis to slip at a plane of cleavage in the skin, which is known as the Nikolsky sign. How the exfoliative toxins produce epidermal splitting has not been fully elucidated. [35]
The most feared manifestation of S aureus toxin production is toxic shock syndrome (TSS). Although first described in children, it was most frequently associated with women using tampons during menstruation. Since the early 1990s, at least half of the cases have not been associated with menstruation. The syndrome is associated with strains that produce the exotoxin TSST-1, but strains that produce enterotoxins B and C may cause 50% of cases of nonmenstrual TSS. These toxins are superantigens, T-cell mitogens that bind directly to invariant regions of major histocompatibility complex class II molecules, causing an expansion of clonal T cells, followed by a massive release of cytokines. This cytokine release mediates the TSS; the resultant pathophysiology mimics that of endotoxic shock.
CA-MRSA
In a worldwide trend, [36] the proportion of infections caused by CA-MRSA has increased. [37] Initially noted in tertiary care centers, these infections are now increasingly common in the community. [38, 39] Resistance to methicillin confers resistance to all penicillinase-resistant penicillins and cephalosporins. This high level of resistance requires the mec gene that encodes penicillin-binding protein 2a. This protein has decreased binding affinity for most penicillins and cephalosporins. Methicillin resistance has a wide variety of phenotypic expression. Heterogeneous resistance, recognized in the first clinical isolates described, is the typical phenotype. In this case, all cells carry the genetic markers of resistance but only a small fraction of them express the phenotype. Homogenous resistance is less frequent, with a single population of cells that are inhibited only through high concentrations of antibiotics.
Methicillin-resistant S aureus (MRSA) was initially described in hospitalized populations. [40] University affiliation and greater number of beds were institutional risk factors. In pediatric centers, number of beds, region, and metropolitan population correlated with increased risk.
Since 1996, more patients with CA-MRSA have been described. The strains isolated from these patients are different from typical nosocomial organisms in their susceptibility patterns and in their PFGE characteristics. A clonal population, designated USA-300, has become the predominant circulating organism in most communities. [41, 42] Many of these strains produce the Panton-Valentine leukocidin, which is associated with a tendency to produce abscesses, invasiveness, thrombogenesis, and morbidity and mortality. [43, 44, 45, 46]
A 2012 report corroborates earlier reports which describe an increased prevalence of USA300 strains causing invasive infections. [47] High levels of antibody to the leukocidin are not protective, [48] however, so the role of this and other virulence factors and immunomodulatory bacterial products remains under investigation. [49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65]
A dose-dependent increase in risk of infection due to CA-MRSA with exposure to antibiotics has been reported. [66]
More recently, S aureus that is intermediately resistant to vancomycin has been reported in 2 hospitalized patients, which suggests that full resistance to vancomycin may eventually emerge, [67] although that has not yet occurred in the pediatric population. [68] Although the possibility of interspecies transfer of vancomycin-resistance genes from vancomycin-resistant Enterococcus was originally considered as the cause of this phenomenon, none of the species isolated have carried vanA, vanB, vanC1, vanC2, or vanC3 genes. Of note, the clinical isolates with intermediate resistance to vancomycin were from patients who had undergone prolonged vancomycin therapy for MRSA. Morphologically, these isolates were found to have increased extracellular material associated with the cell wall that may have been selected for during a prolonged antibiotic course. Some virulence genes appear to be linked to decreased susceptibility to vancomycin. [69, 70]
Most recently, the emergence of newly described mec genes and chromosomal cassettes from bovine sources has been described. [71, 72] Thus, the issue of antimicrobial resistance in S aureus continues to evolve. [73]
Epidemiology
United States statistics
Numbers of both community-associated and hospital-acquired infections have increased in the past 20 years. From 1990-1992, data from the National Nosocomial Infections Surveillance System for the Centers for Disease Control and Prevention (CDC) revealed that S aureus was the most common cause of nosocomial pneumonia and operative wound infections and the second most common cause of nosocomial bloodstream infections.
An analysis of laboratory-confirmed MRSA cases in the Active Bacterial Core Surveillance database (which covers 9 geographic regions and represents some 4.4 million children younger than 18 years of age) indicates that community-acquired invasive MRSA infection is increasing among children, particularly among Black children and infants younger than 90 days of age. The incidence of community-acquired MRSA increased from 1.1 case per 100,000 children in 2005 to 1.7 cases per 100,000 in 2010. The yearly increase in incidence, adjusted for race and age, was 10.2%. The adjusted incidence of invasive MRSA among Black children was 6.7 cases per 100,000 in 2010, compared with 1.6 cases per 100,000 for children of other races. [74, 75]
An analysis of 148 consecutive patients with acute musculoskeletal S aureus infection (111 with MSSA and 37 with MRSA) found that the proportion of pediatric MRSA infections increased from 11.8% to 34.8% between 2001 and 2009, resulting in longer mean hospitalizations (13 vs 8 days). [76] . In addition, children with MRSA infection more often required surgical procedures (38% vs. 15%), experienced more infection-related complications (24% vs 6%), and were more often admitted to the intensive care unit (16% vs 3%).
Frequency of antibiotic resistance: In a disturbing trend, antibiotic resistance among these isolates has increased because of antibiotic pressure. Currently, less than 5% of clinical isolates remain sensitive to penicillin. Resistance to penicillin was reported as early as 1942 and is mediated by beta-lactamase, a serine protease that hydrolyzes the lactam ring. In the 1980s, MRSA emerged as a prominent hospital-based infection; consequently, the use of vancomycin increased. A CDC survey revealed that the proportion of methicillin-resistant isolates with sensitivity only to vancomycin increased from 22.8% in 1987 to 56.2% in 1997. [77] Scattered reports of vancomycin resistance have been noted. [78] Community-onset MRSA infections are increasingly common, [79, 80] although geographic variations persist. [81] Data suggest that the incidence of MRSA infection may have peaked. [82, 83]
A study by Sutter et al analyzed 41,745 S aureus isolates from 39,207 pediatric patients and found that like recent trends seen in adults, the proportion of pediatric S aureus infections secondary to MRSA appear to be decreasing, as is variability in US geographical resistance rates. The authors also added S aureus susceptibility to clindamycin declined from 90% to 83% over a 10-year period. [84]
A large population based case-control study by Smit et al indicated that diabetes patients are almost three times more likely to contract community-acquired Staphylococcus aureus bacteremia, with the risk escalating with worsening disease. [85, 86]
International statistics
The USA300 lineage of community-associated MRSA has become widespread in Latin America. [87] Incidence of staphylococcal skin and complicated infections among children in the United Kingdom has increased over the past decade or so as well, [88, 89] although the incidence of community-associated MRSA blood stream infection in the United Kingdom is less than that seen in the United States. [90] Other strains of community-associated MRSA have been described in Canada, [91, 92, 93, 94] Australia, Asia, [95, 96] and elsewhere in Europe. [97, 98, 99, 100, 101, 102]
Sex-related demographics
The male-to-female ratio of skeletal infections is 2:1, mostly because boys are more likely to experience traumatic events.
Prognosis
Morbidity/mortality
Morbidity and mortality from S aureus infection widely vary depending on the clinical entity. Although mortality is low in children with scalded skin syndrome, most fatalities are associated with delay in diagnosis.
Morbidity and mortality associated with staphylococcal bacteremia in children seem to be less significant than observed in bacteremic adults. [103, 104]
Complications
S aureus infection may result in pneumonia, bone and joint infection, and infection of the heart valves.
In immunocompromised hosts (eg, patients with cancer who are neutropenic and have a central venous line), 20-30% develop serious complications or fatal sepsis following catheter-related S aureus bacteremia or other S aureus infections. [105]
Community-associated methicillin-resistant S aureus (CA-MRSA) infection is more serious and is associated with thrombogenesis. It is becoming endemic in many pediatric and neonatal intensive care units. [106, 107, 108, 109]
Multiple brain abscesses have been reported in premature infants. [110, 111]
S aureus infection complicating influenza infection is associated with significant morbidity and mortality. [112]
-
Posteroanterior chest radiograph of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing borderline cardiomegaly, multiple nodular infiltrates, and bilateral pleural effusions.
-
Lateral chest radiograph of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing borderline cardiomegaly, multiple nodular infiltrates, and bilateral pleural effusions.
-
CT scan of the thorax (mediastinal windows) of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing bilateral pleural effusions.
-
CT scan of the thorax (lung windows) of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, demonstrating multiple nodular infiltrates (some with cavitation) consistent with septic emboli, volume loss on the right, and pleural effusions.
-
CT scan of the thorax (lung windows) of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing multiple nodular infiltrates (some with cavitation) consistent with septic emboli, volume loss on the right, and pleural effusions.
-
CT scan of the thorax (lung windows) of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing multiple nodular infiltrates (some with cavitation) consistent with septic emboli, volume loss on the right, and pleural effusions.
-
CT scan of the thorax (lung windows) of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing multiple nodular infiltrates (some with cavitation) consistent with septic emboli, volume loss on the right, and pleural effusions.
-
Follow-up CT scan of the thorax (lung windows) of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing multiple nodular infiltrates (some with cavitation) consistent with septic emboli, volume loss on the right, and pleural effusions.
-
Follow-up CT scan of the thorax (lung windows) of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing multiple nodular infiltrates (some with cavitation) consistent with septic emboli and evolution of the lesions over time.
-
Follow-up CT scan of the thorax (lung windows) of a 15-year-old with staphylococcal endocarditis and multiple septic emboli, revealing multiple nodular infiltrates (some with cavitation) consistent with septic emboli and evolution of the lesions over time.








