Practice Essentials
Pediatric bacterial meningitis is a serious illness resulting from bacterial meninges infection. It is associated with high morbidity and mortality. Survivors commonly have neurologic sequelae such as hearing loss. Therefore, meticulous attention must be paid to prompt recognition, timely treatment, and close monitoring of children with this disease.
Signs and symptoms
The 3 classic signs and symptoms (less likely in infants and younger children):
-
Fever
-
Headache
-
Meningeal signs (Kernig and Brudzinski)
Signs and symptoms in neonates and infants:
-
Poor feeding
-
Lethargy
-
Irritability
-
Apathy
-
Shrill cry
-
Inconsolibility and increased crying when being held (paradoxical irritability)
-
Fever
-
Hypothermia
-
Seizures
-
Bulging fontanelle
-
Hypotonia
-
Pallor
-
Shock
-
Apnea
-
Jaundice
-
Hypoglycemia
-
Intractable metabolic acidosis
Signs and symptoms in children:
-
Fever (generally present, although some severely ill children present with hypothermia)
-
Nuchal rigidity
-
Photophobia
-
Headache
-
Alterations of the sensorium
-
Irritability
-
Lethargy
-
Anorexia
-
Nausea
-
Vomiting
-
Seizures
-
Coma
See Clinical Presentation for more specific information on the signs and symptoms of pediatric bacterial meningitis.
Diagnosis
Definitive diagnosis is based on the following:
-
Bacteria isolated from the CSF obtained via lumbar puncture:
- CSF bacterial culture
- CSF molecular assays (polymerase chain reaction [PCR] or other nucleic acid amplification tests (NAAT])
-
Meningeal inflammation demonstrated by increased pleocytosis, elevated protein level, and low glucose level in the CSF.
Bacterial meningitis score
Components of the bacterial meningitis score [1] are as follows:
-
Positive CSF Gram stain
-
CSF absolute neutrophil count 1000/µL or higher
-
CSF protein level 80 mg/dL or higher
-
Peripheral blood absolute neutrophil count 10,000/µL or higher
-
History of seizure before or at the time of presentation
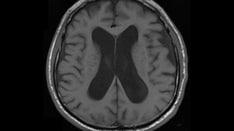
Specific hematologic, radiographic (e.g., computed tomography [CT] and magnetic resonance imaging [MRI]), and other studies assist in diagnosis. CT and MRI may reveal ventriculomegaly and sulcal effacement (see the image below), which can support the diagnosis.
However, a definitive diagnosis requires a lumbar puncture to detect bacteria and evidence of meningeal inflammation.
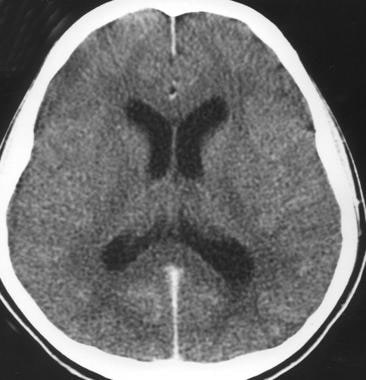 Acute bacterial meningitis. This axial nonenhanced CT scan shows mild ventriculomegaly and sulcal effacement.
Acute bacterial meningitis. This axial nonenhanced CT scan shows mild ventriculomegaly and sulcal effacement.
See Workup for more specific information on testing and imaging modalities for pediatric bacterial meningitis.
Management
See the list below:
- Initial assessment and stabilization of airway, breathing, and circulation
- Manage fluids and electrolytes to ensure appropriate blood pressure and cerebral perfusion.
- Empiric Antibiotics:
If the cause is unknown, empiric agents can be based on the child’s age, as follows:
-
< 30 days: IV ampicillin (for Listeria monocytogenes) plus an IV third-generation cephalosporin (i.e., cefotaxime [where available], ceftazidime; for Group B streptococcus, Enterobacteriaceae)or IV ampicillin plus an IV aminoglycoside (in places with a high incidence of extended-spectrum beta-lactamase-producing Enterobacteriaceae).
-
30-60 days: An IV cephalosporin (i.e., ceftriaxone) because Streptococcus pneumonia and Neisseria meningitis may occur in this age range, plus vancomycin (for resistant Streptococcus pneumoniae and/or Staphylococcus aureus); consider ampicillin if suspicion for Listeria monocytogenes.
-
In older children, a cephalosporin (Streptococcus pneumoniae, Neisseria meningitis, and Haemophilus influenza) plus vancomycin (must be added secondarily to the possibility of resistant S pneumonia and Staphylococcus aureus).
-
If there is suspicion of HSV meningoencephalitis at the time of presentation, consider adding acyclovir for empiric coverage.
Pathogen-Specific Treatment:
Definitive antibiotic therapy should be guided by culture and susceptibility results.
-
S agalactiae (GBS) – penicillin G or ampicillin
-
Listeria monocytogenes – ampicillin or penicillin G +/- an aminoglycoside
-
Streptococcus pneumoniae – penicillin, third generation cephalosporin or vancomycin (specifics guided by culture sensitivities)
-
Haemophilus influenzae – third generation cephalosporin; consider adjunctive dexamethasone just before/with the first dose of antibiotic.
-
Neisseria meningitidis – third generation cephalosporin; ampicillin or penicillin G may be used if susceptible
-
Enterobacteriaceae – third generation cephalosporin; ampicillin or penicillin G may be used if susceptible
Duration of therapy:
Duration of therapy is pathogen-specific, and per the American Academy of Pediatrics and Infectious Disease Society of America guidelines as follows: [2]
-
Neisseria meningitidis – 5-7 days
-
Haemophilus influenzae – 7-10 days
-
Streptococcus pneumoniae - 10-14 days
-
S agalactiae (GBS) - 14-21 days
-
Aerobic gram-negative bacilli - 21 days or 2 weeks beyond the first sterile culture (whichever is longer)
-
Listeria monocytogenes – 21-28 days
Prevention
Preventive therapy has been shown to reduce mortality and morbidity and consists of the following:
-
Neisseria meningitidis:
Preventive measures are as follows:
- Chemoprophylaxis: Post-exposure prophylaxis is indicated for all close contacts of index cases from 7 days before illness onset, including household contacts, preschool/daycare contacts, direct exposure to index patient’s secretions, and passengers sitting nearby on an airline flight. Chemoprophylaxis options are rifampin, ceftriaxone, and ciprofloxacin; ciprofloxacin and ceftriaxone are more effective against resistant strains of Neisseria meningitidis up to 4 weeks after treatment.
- Immunization: Multiple meningococcal vaccines are available in the US. Vaccination with meningococcal ACY W-135 is routinely recommended for adolescents and young adults, and immunocompromised individuals 2 months and older. There are currently 2 vaccines licensed in the US for meningitis B (Trumenba and Bexsero), the most common cause of meningococcal meningitis. Although the meningococcal B vaccine is not part of routine immunization, it is recommended for people at increased risk, including immunocompromised individuals, and those living in dorms or military barracks, and is required by some colleges.
-
Available meningococcal vaccines in the United States include the following:
- Quadrivalent (ie, A, C, Y, W-135) meningococcal conjugate vaccine (Menactra, Menveo, MenQuadfi) in early- and mid-adolescence and also recommended for high-risk groups
- Pentavalent (ie, A, B, C, W, Y) meningococcal vaccine (Penbraya) is recommended for patients aged 10-25 years
- Meningococcal serogroup B vaccine (Bexsero, Trumenba) may be considered for high-risk individuals, in an outbreak setting, and to protect against serotype B in congregant settings
-
Haemophilus influenzae type b (Hib):
Preventive measures are as follows:
- Chemoprophylaxis: Rifampin chemoprophylaxis indications include
- All household contacts of index case if there is an incompletely immunized child less than 4 years or an immunocompromised child regardless of immunization status.
- Contacts at childcare centers with 2 or more cases of invasive Hib within 60 days.
- Immunization: Multiple monovalent and combination vaccines containing Hib conjugate are available in the US, including ActHIB, PedvaxHIB, Hiberix, Pentacel (Hib and DTaP-IPV), and Vaxelis (Hib, DTaP-IPV, and HepB).
- Chemoprophylaxis: Rifampin chemoprophylaxis indications include
-
Streptococcus pneumoniae:
Preventive measures are as follows:
- Chemoprophylaxis: Prophylaxis with penicillin is only recommended to prevent invasive pneumococcal disease in immunocompromised children with functional or anatomical asplenia.
- Immunization: The 13-valent pneumococcal conjugate vaccine (PCV13) is routinely recommended for all children 2-59 months old. New 15-valent and 20-valent pneumococcal conjugate vaccines have also been approved for children. The 23-valent pneumococcal polysaccharide vaccine (PPSV23) is only recommended for children at increased risk, including those with asplenia, HIV infection, cochlear implants, CSF leak, and other immunocompromising conditions.
See Treatment and Medication for more specific information on pharmacologic and other therapies for pediatric bacterial meningitis.
Background
Pediatric bacterial meningitis is a life-threatening illness resulting from bacterial meninges infection. Because bacterial meningitis in the neonatal period has unique epidemiologic and etiologic features, so it will be discussed separately in this article
Beyond the neonatal period, the 3 most common organisms that cause acute bacterial meningitis are Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b (Hib).
Since the routine use of Hib, conjugate pneumococcal, and conjugate meningococcal vaccines in the United States, the incidence of meningitis has dramatically decreased. Although S pneumoniae is now the leading cause of community-acquired bacterial meningitis in the United States, the rate of invasive pneumococcal disease has decreased by 98% after the introduction of the pneumococcal vaccine, PCV 7 in 2000, and PCV 13 in 2010. The pneumococcal vaccine has also resulted in a > 80 % decline in resistant S pneumoniae meningitis cases. The incidence of disease caused by S pneumoniae is highest in children aged 1-23 months and adults older than 60. Similarly, the rates of meningococcal meningitis have dramatically decreased since the 1990s. The incidence of invasive Haemophilus influenza disease has declined by > 99%.
Predisposing factors include respiratory infection, otitis media, mastoiditis, head trauma, hemoglobinopathy, human immunodeficiency virus (HIV) infection, and other immune deficiency states.
Meningitis is a life-threatening illness and leaves some survivors with significant sequelae. Therefore, meticulous attention must be paid to the appropriate treatment and monitoring of these patients. Patients require hospitalization for antibiotic therapy and appropriate support. Adequate fluid administration is necessary to maintain perfusion, especially cerebral perfusion. Fluid restrictions (to prevent cerebral edema) may be more harmful because patients may be under resuscitated. Antibiotics must be promptly administered.
Pathophysiology
The first step in infection is establishing colonization in the nasopharynx by the three common pathogens responsible for bacterial meningitis, Streptococcus pneumoniae, Neisseria meningitis, and Haemophilus influenzae. These encapsulated bacteria can evade the local immune response and access the bloodstream. Bacteria reach the subarachnoid space via the hematogenous route. Bacteria may directly reach the meninges in patients with a parameningeal focus of infection.
Once pathogens enter the subarachnoid space, an intense host inflammatory response is triggered by lipoteichoic acid and other bacterial cell wall products produced from bacterial lysis. This response is mediated by stimulating macrophage-equivalent brain cells that produce cytokines and other inflammatory mediators. This cytokine activation then initiates several processes that ultimately cause damage in the subarachnoid space, culminating in neuronal injury and apoptosis.
Interleukin (IL)–1, tumor necrosis factor-alpha (TNF-a), and enhanced nitric oxide production are critical in triggering an inflammatory response and ensuing neurologic damage. Infection and inflammatory response later affect penetrating cortical vessels, resulting in swelling and proliferation of the endothelial cells of arterioles. A similar process can involve the veins, causing mural thrombi and obstruction of flow. The result is an increase in intracellular sodium and intracellular water.
The development of brain edema further compromises cerebral circulation, and this effect can result in increased intracranial pressure (ICP) and uncal herniation. Increased secretion of antidiuretic hormone (ADH), resulting in the syndrome of inappropriate antidiuretic hormone secretion (SIADH), occurs in most patients with meningitis and causes further retention of free water. These factors contribute to the development of focal or generalized seizures.
Severe brain edema also causes midline structures to shift caudally and become entrapped in the tentorial notch or foramen magnum. Caudal shifts produce herniation of the parahippocampal gyri, cerebellum, or both. These intracranial changes appear clinically as an alteration of consciousness and postural reflexes. Caudal displacement of the brainstem causes palsy of the third and sixth cranial nerves. If untreated, these changes result in decortication or decerebration and can progress rapidly to respiratory and cardiac arrest.
Neonatal meningitis
Bacteria from the maternal genital tract colonize the neonate after rupture of membranes, and specific bacteria, such as group B streptococci (GBS), enteric gram-negative rods, and Listeria monocytogenes, can reach the fetus transplacentally and cause infection. Furthermore, newborns can also acquire bacterial pathogens from their surroundings, and several host factors facilitate a predisposition to bacterial sepsis and meningitis.
Bacteria reach the meninges via the bloodstream and cause inflammation. After arriving in the central nervous system (CNS), bacteria spread from the longitudinal and lateral sinuses to the meninges, the choroid plexus, and the ventricles.
IL-1 and TNF-a also mediate local inflammatory reactions by inducing phospholipase A2 activity, initiating the production of platelet-activating factor and the arachidonic acid pathway. This process results in the production of prostaglandins, thromboxanes, and leukotrienes. Activation of adhesion-promoting receptors on endothelial cells by these cytokines attracts leukocytes, and the release of proteolytic enzymes from the leukocytes results in altered blood-brain permeability, activation of the coagulation cascade, brain edema, and tissue damage.
Inflammation of the meninges and ventricles produces a polymorphonuclear response, an increase in cerebrospinal fluid (CSF) protein content, and utilization of glucose in CSF. Inflammatory changes and tissue destruction in the form of empyema and abscesses are more pronounced in gram-negative meningitis. Thick inflammatory exudate causes blockage of the aqueduct of Sylvius and other CSF pathways, resulting in both obstructive and communicating hydrocephalus.
Etiology
Causes in different age groups
Neonates
Bacteria are often acquired from the maternal vaginal flora. Gram-negative enteric flora and GBS are the dominant pathogens. In premature newborns who receive multiple antibiotics, those on hyperalimentation, and those who undergo various surgical procedures, Staphylococcus epidermidis and Candida species are uncommon but are reported in greater frequency in neonates. L monocytogenes is another well-known but fairly uncommon causative pathogen.
Early-onset GBS meningitis occurs during the first 7 days of life as a consequence of maternal colonization and the absence of protective antibody in the neonate; it is often associated with obstetric complications. The disease is seen most often in premature or low-birth-weight babies. Pathogens are acquired before or during the birth process.
Late-onset meningitis is defined as disease occurring after 7 days of life. Causes include perinatally acquired and nosocomial pathogens. Streptococcus agalactiae (GBS) is classified into 5 distinct serotypes: Ia, Ib, Ic, II, and III. Although these serotypes occur with almost equal frequency in the early onset of disease, serotype III causes 90% of late-onset disease.
Use of respiratory equipment in the nursery increases the risk of infection caused by Serratia marcescens,Pseudomonas aeruginosa, and Proteus species. Invasive devices predispose infants to the infections caused by S epidermidis and Pseudomonas, Citrobacter, and Bacteroides species.
Infection with Citrobacter diversus, Citrobacter koseri, Salmonella species, and Proteus species, though uncommon, carries a high mortality. These patients often develop brain abscesses, particularly those with Citrobacter, in whom meningitis produces brain abscesses in 80-90% of cases.
A cohort study of infants < 90 days of age with bacterial meningitis by Ouchenir et al reported that E coli (33%) and GBS (31%) remain the most common causes of bacterial meningitis in the first 90 days of life. The study also concluded that if there is evidence for Gram-negative meningitis, a third-generation cephalosporin (plus ampicillin for at least the first month), can be considered. [3]
Infants and children
In children older than 4 weeks, S pneumoniae and N meningitidis are the most common etiologic agents. Hib has essentially disappeared in countries where the conjugate vaccine is routinely used.
Causative organisms
Streptococcus pneumoniae
S pneumoniae is a gram-positive, lancet-shaped diplococcus that is the leading cause of meningitis. Of the 100 serotypes, numbers 1, 3, 6, 7, 14, 19, and 23 are the ones most often associated with bacteremia and meningitis. Children of any age may be affected, but the incidence and severity are highest in very young and elderly persons.
In patients with recurrent meningitis, predisposing factors are anatomic defects, asplenia, and primary immune deficiency. Often, the history includes recent or remote head trauma. This organism also has a predilection for causing meningitis in patients with sickle cell disease, other hemoglobinopathies, and functional asplenia. Immunity is type-specific and long-lasting.
S pneumoniae colonizes the upper respiratory tract of healthy individuals; however, disease often is caused by a recently acquired isolate. Transmission is person-to-person, usually via direct contact; secondary cases are rare. The incubation period is 1-7 days, and infections are more common in winter, when viral respiratory disease is prevalent. The disease often results in sensorineural hearing loss, hydrocephalus, and other central nervous system (CNS) sequelae. Prolonged fever despite adequate therapy is common with S pneumoniae meningitis.
Effective antimicrobial therapy can eradicate the organism from nasopharyngeal secretions within 24 hours. However, pneumococci have developed resistance to a variety of antibiotics; this development is seen worldwide. Rates of resistance to penicillin range from 10% to 60%. Multicenter surveillance of pneumococci isolated from the cerebrospinal fluid (CSF) has found resistance rates of 20% to penicillin and 7% to ceftriaxone.
Penicillin resistance in pneumococci is due to alterations in enzymes necessary for growth and repair of the penicillin-binding proteins; thus, beta-lactamase inhibitors offer no advantage. Penicillin-resistant pneumococci are often resistant to trimethoprim-sulfamethoxazole, tetracyclines, chloramphenicol, and macrolides. However, selected third-generation cephalosporins (eg, cefotaxime and ceftriaxone) do exhibit activity against most penicillin-resistant pneumococcal isolates.
At present, all pneumococcal isolates remain susceptible to vancomycin and various oxazolidinones. Several of the fluoroquinolones (eg, levofloxacin), though contraindicated in children, have excellent activity against most pneumococci and achieve adequate CNS penetration.
Tolerance, a trait distinct from resistance, is the term used to characterize bacteria that stop growing in the presence of antibiotic yet do not lyse and die. Pneumococci that are tolerant of penicillin and vancomycin have been described in literature, and a subsequent link to recrudescent meningitis was described in 1 child. The overall incidence and clinical impact of such bacterial strains are unknown. However, the possibility of tolerance should be kept in mind in cases of recurrent pneumococcal meningitis.
Neisseria meningitidis
N meningitidis is a gram-negative, kidney bean–shaped organism that is frequently found intracellularly. Organisms are grouped serologically on the basis of capsular polysaccharide; A, B, C, D, X, Y, Z, 29E, and W-135 are the pathogenic serotypes. In developed countries, serotypes B, C, Y, and W-135 account for most childhood cases. Group A strains are most prevalent in developing countries and have resulted in epidemics of meningococcal meningitis throughout the world, as well as outbreaks in military barracks.
A systematic review by Sridhar et al indicated that in most countries around the world, the annual incidence of invasive N meningitidis serogroup B between January 2000 and March 2015 was less than 2 cases per 100,000 people, although the incidence was higher in Australia, Europe, North America, and South America. [4]
The upper respiratory tract frequently is colonized with meningococci, and transmission is person-to-person via direct contact with infected droplets of respiratory secretions, often from asymptomatic carriers. The incubation period is generally less than 4 days (range, 1-7 days).
Most cases occur in infants aged 6-12 months; a second, lower peak occurs among adolescents. A petechial or purpuric rash frequently is seen. Mortality is significant in patients who have a rapidly progressive fulminant form of the disease. Normocellular CSF also has been reported in patients with meningococcal meningitis. Most deaths occur within 24 hours of hospital admission in patients who have features associated with poor prognosis, such as the following:
-
Hypotension
-
Shock
-
Neutropenia
-
Extremes of age
-
Petechiae and purpura of less than 12 hours’ duration
-
Disseminated intravascular coagulation (DIC)
-
Acidosis
-
Presence of the organism in white blood cells (WBCs) on peripheral smear
-
Low erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level
-
Serogroup C disease
Higher rates of fatality and physical sequelae (eg, scarring and amputation) are reported in survivors of serogroup C disease. Long-term sequelae are rare in patients who have an uneventful hospital course.
Haemophilus influenzaetype b (Hib)
Hib is a pleomorphic gram-negative rod whose shape varies from a coccobacillary form to a long curved rod. Hib meningitis occurs primarily in children who have not been immunized with Hib vaccine; 80-90% of cases occur in children aged 1 month to 3 years. By age 3 years, a significant number of nonimmunized children acquire antibodies against the capsular polyribophosphate of Hib, which are protective.
The mode of transmission is person-to-person via direct contact with infected droplets of respiratory secretions. The incubation period generally is less than 10 days. Current mortality is less than 5%. Most fatalities occur during the first few days of the illness.
Plasmid-mediated resistance to ampicillin due to the production of beta-lactamase enzymes by bacterium is increasingly being reported: 30-35% of Hib isolates are now ampicillin-resistant. As many as 30% of cases may have subtle long-term sequelae. Administration of dexamethasone early in treatment reduces morbidity and sequelae.
Listeria monocytogenes
L monocytogenes causes meningitis in newborns, immunocompromised children, and pregnant women. The disease also has been associated with the consumption of contaminated foods (eg, milk and cheese). Most cases are caused by serotypes Ia, Ib, and IVb. Signs and symptoms in patients with listerial meningitis tend to be subtle, and diagnosis often is delayed. In the laboratory, this pathogen can be misidentified as a diphtheroid or a hemolytic streptococcus.
Other organisms
S epidermidis and other coagulase-negative staphylococci frequently cause meningitis and CSF shunt infection in patients with hydrocephalus or those who have undergone neurosurgical procedures. Immunocompromised children can develop meningitis caused by Pseudomonas, Serratia,Proteus, and diphtheroids.
Risk factors
Risk factors for bacterial meningitis include the following:
-
Age
-
Low family income
-
Attendance at day care
-
Head trauma
-
Splenectomy
-
Chronic disease
-
Children with facial cellulitis, periorbital cellulitis, sinusitis, and septic arthritis have an increased risk of meningitis.
-
Maternal infection and pyrexia at the time of delivery are associated with neonatal meningitis
Use of Hib and pneumococcal vaccines decreases the likelihood of infection from these agents.
Epidemiology
United States statistics
The advent of vaccine has changed the incidence of pediatric bacterial meningitis. Before the routine use of the pneumococcal conjugate vaccine, the incidence of bacterial meningitis in the United States was about 6000 cases per year; roughly half of these were in pediatric patients (≤18 years). N meningitidis caused about 4 cases per 100,000 children (aged 1-23 months). The rate of S pneumoniae meningitis was 6.5 cases per 100,000 children (aged 1-23 months). Today, disease caused by H influenzae, S pneumoniae, and N meningitidis is much less common.
The advent of universal Hib vaccination in developed countries has led to the elimination of more than 99% of invasive disease. Protection continues even when Hib is coadministered with other vaccines. Just as important, the vaccine continues to confer immunity into later childhood.
A similar effect occurs with pneumococcal vaccine. Given at ages 2, 4, and 6 months, this vaccine has reduced invasive disease by more than 90%. Age groups most affected are those younger than 2 years and those aged 2-5 years. Nearly half of cases of pneumococcal disease are caused by nonvaccine serotypes. [5]
Vaccine for Neisseria, however, has not been efficacious in younger children. This is due to poor immunogenic response. Current recommendations target immunization for children older than 2 years and high-risk patients with asplenic and terminal complement deficiencies. In addition, young adults living in close quarters, such as dormitories or military barracks, will benefit.
A study analyzing reported cases of bacterial meningitis among residents in 8 surveillance areas of the Emerging Infections Programs Network during 1998-2007 found a 31% decrease in meningitis cases during this period and an increase in median patient age from 30.3 years in 1998-1999 to 41.9 years in 2006-2007; the case fatality rate did not change significantly. [6] Overall, approximately 4100 cases of bacterial meningitis occurred annually in the United States from 2003 to 2007, with approximately 500 deaths. [6]
A study that reported on the epidemiology of infant meningococcal disease in the United States from 2006 to 2012 found that an estimated 113 cases of culture-confirmed meningococcal disease occurred annually among infants aged < 1 year in the United States from 2006 through 2012, for an overall incidence of 2.74 per 100,000 infants. Among these cases, an estimated 6 deaths occurred. Serogroup B was responsible for 64%, serogroup C for 12%, and serogroup Y for 16% of infant cases. Based on the expanded data collection forms, a high proportion of infant cases (36/58, 62%) had a smoker in the household and the socioeconomic status of the census tracts where infant meningococcal cases resided was lower compared with the other Active Bacterial Core surveillance areas and the United States as a whole. [7]
The incidence of neonatal bacterial meningitis is 0.25-1 case per 1000 live births (0.15 case per 1000 full-term births and 2.5 cases per 1000 premature births). Approximately 30% of newborns with clinical sepsis have associated bacterial meningitis.
After the initiation of intrapartum antibiotics in 1996, the national incidence of early-onset GBS infection decreased substantially, from approximately 1.8 cases per 1000 live births in 1990 to 0.32 case per 1000 live births in 2003.
International statistics
Worldwide, the use of H influenzae type B and pneumococcal vaccines is increasing at a rate faster than that observed with the use of hepatitis B vaccines. [8]
In a survey by the Hib and Pneumococcal Working Group, the incidence of meningitis in 2000 varied in different regions of the world. The overall incidence of pneumococcal meningitis was 17 cases per 100,000, with the highest incidence in Africa, at 38 cases per 100,000, and the lowest incidence in Europe, at 6 cases per 100,000. [9] The overall death rate was 10 cases per 100,000. The death rate was highest in Africa, at 28 cases per 100,000, and lowest in Europe and Western Pacific regions, at 3 cases per 100,000.
A similar trend was identified for Hib meningitis. [10] The overall incidence of Hib meningitis in 2000 was 31 cases per 100,000. The African region had the highest rate, at 46 cases per 100,000, and Europe had the lowest, at 13 cases per 100,000. The overall death rate was 13 cases per 100,000. The highest death rate was in Africa, at 31 cases per 100,000, and the lowest was in Europe, at 4 cases per 100,000.
Age-, sex-, and race-related demographics
Pediatric bacterial meningitis is most common in children younger than 4 years, with a peak incidence in those aged 3-8 months.
Male infants have a higher incidence of gram-negative neonatal meningitis. Female infants are more susceptible to L monocytogenes infection. S agalactiae (GBS) affects both sexes equally.
Bacterial meningitis occurs more frequently in black, Native American, and Hispanic children; this is thought to be related to socioeconomic rather than racial factors.
Prognosis
Mortality and morbidity depend on the infectious agent, the age of the child, the child’s general health, and the promptness of diagnosis and treatment. Despite improvements in antibiotic and supportive therapy, death and complication rates remain significant.
Overall mortality for bacterial meningitis is 5-10% and varies according to the causative organism and the patient’s age. In neonates, mortality is 15-20%, whereas in older children, it is 3-10%. Of the meningitides caused by the most common pathogens, S pneumoniae meningitis has the highest mortality, at 26.3-30%; Hib meningitis has the next highest, at 7.7-10.3%; and N meningitidis has the lowest, at 3.5-10.3%.
As many as 30% of children have neurologic sequelae. This rate varies by organism, with S pneumoniae being associated with the highest rate of complications. One study indicated that the complication rate from S pneumoniae meningitis was essentially the same for penicillin-sensitive strains as for penicillin-resistant strains; this study also showed that dexamethasone did not improve outcomes. [11]
Prolonged or difficult-to-control seizures, especially after hospital day 4, are predictors of a complicated hospital course with serious sequelae. On the other hand, seizures that occur during the first 3 days of illness usually have little prognostic significance.
Approximately 6% of affected infants and children show signs of DIC and endotoxic shock. These signs are indicative of a poor prognosis.
Studies have documented the development of profound bilateral hearing loss, which may occur in as many as 4% of all bacterial meningitis cases. Sensorineural hearing loss is one of the most frequent problems. Children at greatest risk for hearing loss include those with evidence of increased ICP, those with abnormal findings on computed tomography (CT), males, those with low CSF glucose levels, those with S pneumoniae infection , and those with nuchal rigidity. [11, 12]
Because many of the children affected are very young and lack mature cognitive and motor skills, some of the sequelae may not be recognized for years. In a study that followed children who recovered from meningitis for 5-10 years, 1 of every 4 school-aged meningitis survivors had either serious and disabling sequelae or a functionally important behavior disorder or neuropsychiatric or auditory dysfunction that impaired their performance in school.
For tuberculous meningitis, morbidity and mortality are related to the stage of the disease. The rate of significant morbidity is 30% for stage I, 56% for stage II, and 94% for stage III.
Patient Education
Because of the high incidence of sequelae, parents should be cautioned from the beginning that even with appropriate medical care, the child may have some complications. Respond promptly to parents’ concerns with adequate documentation.
Careful neurologic examination and visual and hearing screening tests (brainstem evoked potentials) should be obtained and reviewed with parents so that parents are aware of any deficits. Early detection of deficits should result in initiating appropriate physical and occupational therapy and in acquiring other devices or modalities required by the patient to achieve the maximum possible benefit.
-
Acute bacterial meningitis. This axial nonenhanced CT scan shows mild ventriculomegaly and sulcal effacement.
-
Acute bacterial meningitis. This axial T2-weighted MRI shows only mild ventriculomegaly.
-
Acute bacterial meningitis. This contrast-enhanced, axial T1-weighted MRI shows leptomeningeal enhancement (arrows).