Practice Essentials
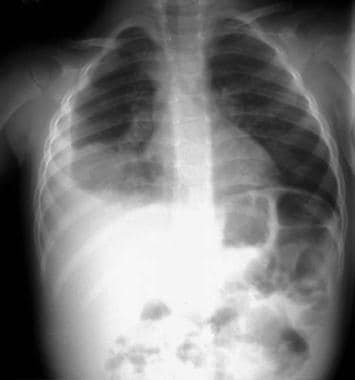
Pediatric pneumonia is responsible for the deaths of more than 800,000 young children worldwide each year, according to the United Nations Children's Fund (UNICEF). [1] These deaths occur almost exclusively in children with underlying conditions, such as chronic lung disease of prematurity, congenital heart disease, and immunosuppression. Although most fatalities occur in developing countries, pneumonia (see the image below) remains a significant cause of morbidity in industrialized nations.
Signs and symptoms
Pneumonia can occur at any age; nevertheless, it is more common in younger children. Pneumonia accounts for 13% of all infectious illnesses in infants younger than 2 years of age.
Newborns with pneumonia commonly present with poor feeding and irritability, as well as tachypnea, retractions, grunting, and hypoxemia. Infections with group B Streptococcus, Listeria monocytogenes, or gram-negative rods (eg, Escherichia coli, Klebsiella pneumoniae) are common causes of bacterial pneumonia. Group B streptococci infections are most often transmitted to the fetus in utero. The most commonly isolated virus is respiratory syncytial virus (RSV).
Cough is the most common symptom of pneumonia in infants, along with tachypnea, retractions, and hypoxemia. These may be accompanied by congestion, fever, irritability, and decreased feeding. Viruses are the most common cause of pediatric pneumonia. Streptococcus pneumoniae is the most common bacterial pathogen in infants aged 1-3 months.
Adolescents experience symptoms similar to those in younger children. They may have other constitutional symptoms, such as headache, pleuritic chest pain, and nonspecific abdominal pain. Mycoplasma pneumoniae is the most frequent cause of pneumonia among older children and adolescents.
See Clinical Presentation for more detail.
Diagnosis
The signs and symptoms of pneumonia are often nonspecific and vary widely based on the child's age and the infectious organisms involved.
Observing the child’s respiratory effort during a physical examination is an important first step in diagnosing pneumonia. The World Health Organization (WHO) respiratory rate thresholds for identifying children with pneumonia are as follows:
-
Children younger than 2 months: Greater than or equal to 60 breaths/min
-
Children aged 2-12 months: Greater than or equal to 50 breaths/min
-
Children aged 1-5 years: Greater than or equal to 40 breaths/min
Be aware that the WHO definition requires only cough and tachypnea on physical examination. [2]
Assessment of oxygen saturation by pulse oximetry should be performed early in the evaluation when respiratory symptoms are present. Cyanosis may be present in severe cases. Capnography may be useful in the evaluation of children with potential respiratory compromise.
Other diagnostic tests may include the following:
-
Auscultation by stethoscope
-
Cultures
-
Serology
-
Complete blood cell count (CBC)
-
Chest radiography
-
Ultrasonography
Current data show that point-of-care ultrasonography helps accurately diagnose most cases of pneumonia in children and young adults. Ultrasonography may eventually replace radiographs for diagnosis. [3]
See Workup for more detail.
Management
Initial priorities in children with pneumonia include the identification and treatment of respiratory distress, hypoxemia, and hypercarbia. Grunting, flaring, severe tachypnea, and retractions should prompt immediate respiratory support. Children who are in severe respiratory distress should undergo tracheal intubation if they are unable to maintain oxygenation or have decreasing levels of consciousness. Increased respiratory support requirements such as increased inhaled oxygen concentration, positive pressure ventilation, or continuous positive airway pressure (CPAP) are commonly required before recovery begins.
Antibiotics
The majority of children diagnosed with pneumonia in the outpatient setting are treated with oral antibiotics. High-dose amoxicillin is the agent of choice for children with uncomplicated community-acquired pneumonia. Second- or third-generation cephalosporins and macrolide antibiotics such as azithromycin are acceptable alternatives. Combination therapy (ampicillin and either gentamicin or cefotaxime) is typically used in the initial treatment of newborns and young infants.
Hospitalized patients can also usually be treated with a narrow-spectrum penicillin such as ampicillin. The choice of agent and dosing may vary based on local resistance rates (high rates of intermediate or resistant pneumococcus may require higher dosing of ampicillin to surmount the altered penicillin-binding protein that is the cause of resistant pneumococcus). In areas where resistance is very high (>25% of strains being nonsusceptible), a third-generation cephalosporin might be indicated instead. In addition, older children may receive a macrolide to cover for atypical infections.
Although the fluoroquinolones would cover all the common respiratory pathogens of childhood, they are not approved for this indication and have significant potential adverse effects, including short-term tendon damage and long-term impact on antibiotic resistance. They should be reserved for cases in which other therapies have failed and ideally should be used after consultation with an infectious disease specialist with whom other options, or alternative diagnoses, can be considered.
Children who are toxic appearing should receive antibiotic therapy that includes vancomycin (particularly in areas where penicillin-resistant pneumococci and methicillin-resistant Staphylococcus aureus [MRSA] are prevalent) along with a second- or third-generation cephalosporin.
Vaccines
Aside from avoiding infectious contacts (difficult for many families who use daycare facilities), vaccination is the primary mode of prevention. Influenza vaccine is recommended for children aged 6 months and older. The pneumococcal conjugate vaccine (PCV13) is recommended for all children younger than 59 months of age. The 23-valent polysaccharide vaccine (PPV23) is recommended for children aged 24 months and older who are at high risk for pneumococcal disease.
See Treatment and Medication for more detail.
Background
Pneumonia and other lower respiratory tract infections are the leading causes of death worldwide. Because pneumonia is common and is associated with significant morbidity and mortality, promptly diagnosing pneumonia, correctly recognizing any complications or underlying conditions, and appropriately treating patients are all important. Although in developed countries the diagnosis is usually made on the basis of radiographic findings, the World Health Organization (WHO) has defined pneumonia solely on the basis of clinical findings obtained by visual inspection and on the timing of the respiratory rate.
Pneumonia may originate in the lung or may be a focal complication of a contiguous or systemic inflammatory process. Abnormalities of airway patency as well as alveolar ventilation and perfusion occur frequently due to a range of possible mechanisms. These derangements often significantly alter gas exchange and dependent cellular metabolism in the many tissues and organs that determine survival and contribute to quality of life. Recognition, prevention, and treatment of these problems are major factors in the care of children with pneumonia.
One particular form of pneumonia present in the pediatric population, congenital pneumonia, presents within the first 24 hours after birth. For more information, see Congenital Pneumonia.
Other respiratory tract diseases such as croup (laryngotracheobronchitis), bronchiolitis, and bronchitis are beyond the scope of this article and are not discussed further.
Pathophysiology
An inhaled infectious organism must bypass the host's normal nonimmune and immune defense mechanisms in order to cause pneumonia. The nonimmune mechanisms include aerodynamic filtering of inhaled particles based on size, shape, and electrostatic charges; the cough reflex; mucociliary clearance; and several secreted substances (eg, lysozymes, complement, defensins). Macrophages, neutrophils, lymphocytes, and eosinophils carry out the immune-mediated host defenses.
Respiratory tract host defenses
To prevent and minimize injury and invasion by microorganisms and foreign substances, various defense mechanisms have evolved, both systemically and within the respiratory tract. Some mechanisms are nonspecific and are directed against any invasive agent, whereas others are targeted against only microbes or substances with specific antigenic determinants. Many of the defenses are compromised in the fetus and newborn infant. These compromises may result in more frequent breaches and a consequent disruption of normal lung structure and function. [4]
Nonspecific defenses include the glottis and vocal cords, ciliary escalator, airway secretions, migratory and fixed phagocytes, nonspecific antimicrobial proteins and opsonins, and normal relatively nonpathogenic airway flora. Anatomic structures of the upper airway and associated reflexes discourage particulate material from entering. On the other hand, coordinated movement of the microscopic cilia on the tracheal and bronchial epithelia tends to sweep particles and mucus up the airway and away from the alveoli and distal respiratory structures.
Mucoid airway secretions provide a physical barrier that minimizes epithelial adhesion and subsequent invasion by microorganisms. These secretions typically contain complement components, fibronectin, and other proteins that bind to microbes and render them more susceptible to ingestion by phagocytes. Alveolar and distal airway secretions also include whole surfactant, which facilitates opsonization and phagocytosis of pathogens, as well as surfactant-associated proteins A (Sp-A) and D (Sp-D). Both of these latter proteins can modulate phagocytosis, phagocyte production of oxyradicals, and cytokine elaboration.
These secretions also contain direct inhibitory and microbicidal agents, such as iron-binding proteins, lysozymes, and defensins. Typical benign airway commensals, such as alpha-hemolytic streptococci and coagulase-negative staphylococci, occupy mucosal sites and elaborate bacteriocins and other substances that prevent more pathogenic organisms from adhesion, replication, and possible opportunistic invasion.
Newborns typically have sterile respiratory mucosa at birth, with subsequent uncontested colonization by microorganisms from the mother or the environment. Accelerated access to distal respiratory structures and bypass of much of the ciliary escalator occur in infants who require endotracheal intubation. In these infants, increased physical disruption of epithelial and mucosal barriers also occurs. In addition, interventional exposure to high oxygen concentrations, generous airway pressures, and large intrapulmonary gas volumes may interfere with ciliary function and mucosal integrity. The use of less invasive means of respiratory support, such as nasal ventilation, nasal continuous positive airway pressure (CPAP), and nasal cannula (conventional or humidified, high flow), may produce lesser degrees of pulmonary mucosal and parenchymal disruption; however, some disruption is almost always present.
Systemic host defenses
Immunologic defense mechanisms targeted against particular pathogens typically emanate from specifically primed lymphocytes following the presentation of processed antigens by macrophages. These mechanisms include cytotoxic, killer, suppressor, and memory functions; systemic and secretory antibodies; and consequent cascades of cytokines, complement, vasomotor regulatory molecules, hemostatic factors, and other agents. Secretory antibodies are typically multimeric and contain secretory component and J chains that render them more opsonic and more resistant to microbial proteases.
Many of the biochemical cascades triggered by specific immune responses serve to localize microbial invasion, amplify and focus recruitment of phagocytes to the affected sites, and directly disrupt the structural and metabolic integrity of the microbes. The role that these cascades may play in triggering apoptosis (programmed cell death) in host and invader cells is still undergoing exploration.
Secretory antibodies and mucosal lymphoid tissue are absent or minimally functional for the first month of postnatal life. Systemic antibodies may enter pulmonary tissues but usually consist primarily of passively transmitted maternal antibodies. Furthermore, there is reduced transplacental transport of maternal antibodies before 32 weeks' gestation. Specific systemic antibodies can be generated, but many components of the necessary immunologic machinery act relatively sluggishly. Circulating complement components are present at approximately 50% of the concentration found in older children. Nevertheless, components of the alternative pathway are present in sufficient quantities to serve as effective opsonins.
The neonatal granulocyte number frequently decreases in response to early infection (as well as noninflammatory processes such as maternal preeclampsia). On the other hand, the phagocytes that are present often move much more sluggishly to the inflammatory focus, whether they are responding to the presence of a microorganism or inanimate debris. Once at the targeted sites, phagocytes often ingest the invader substances less efficiently, although intracellular microbicidal activities appear normal. Intercellular communication via cytokines and other mediators is blunted.
The net result of these and other developmental aberrations is that the fetal and neonatal inflammatory response is slower, less efficient, and much less focused than in older children. Infection is less likely to be localized and effectively inhibited by host defenses alone. Inflammation from particulate debris and other foreign substances is isolated less effectively, and the injurious effector portions of the inflammatory cascade are much less precisely targeted.
Pathogenesis
Pneumonia is characterized by inflammation of the alveoli and terminal airspaces in response to invasion by an infectious agent introduced into the lungs through hematogenous spread or inhalation. The inflammatory cascade triggers the leakage of plasma and the loss of surfactant, resulting in air loss and consolidation.
The activated inflammatory response often results in targeted migration of phagocytes, with the release of toxic substances from granules and other microbicidal packages and the initiation of poorly regulated cascades (eg, complement, coagulation, cytokines). These cascades may directly injure host tissues and adversely alter endothelial and epithelial integrity, vasomotor tone, intravascular hemostasis, and the activation state of fixed and migratory phagocytes at the inflammatory focus. The role of apoptosis (noninflammatory programmed cell death) in pneumonia is poorly understood.
Pulmonary injuries are caused directly and/or indirectly by invading microorganisms or foreign material and by poorly targeted or inappropriate responses by the host defense system that may damage healthy host tissues as badly as or worse than the invading agent. Direct injury by the invading agent usually results from synthesis and secretion of microbial enzymes, proteins, toxic lipids, and toxins that disrupt host cell membranes, metabolic machinery, and the extracellular matrix that usually inhibits microbial migration.
Indirect injury is mediated by structural or secreted molecules, such as endotoxin, leukocidin, and toxic shock syndrome toxin-1 (TSST-1). Any of these molecules may alter local vasomotor tone and integrity, change the characteristics of the tissue perfusate, and generally interfere with the delivery of oxygen and nutrients and removal of waste products from local tissues. [5, 6]
On a macroscopic level, the invading agents and the host defenses all tend to increase airway smooth muscle tone and resistance, mucus secretion, and the presence of inflammatory cells and debris in these secretions. These materials may further increase airway resistance and obstruct the airways, partially or totally, causing airtrapping, atelectasis, and ventilatory dead space. In addition, disruption of endothelial and alveolar epithelial integrity may allow surfactant to be inactivated by proteinaceous exudate, a process that may be exacerbated further by the direct effects of meconium or pathogenic microorganisms.
In the end, conducting airways offer much more resistance and may become obstructed, alveoli may be atelectatic or hyperexpanded, alveolar perfusion may be markedly altered, and multiple tissues and cell populations in the lung and elsewhere sustain injury that increases the basal requirements for oxygen uptake and excretory gas removal at a time when the lungs are less able to accomplish these tasks.
Alveolar diffusion barriers may increase, intrapulmonary shunts may worsen, and ventilation/perfusion (V/Q) mismatch may further impair gas exchange despite endogenous homeostatic attempts to improve matching by regional airway and vascular constriction or dilatation. Because the myocardium has to work harder to overcome the alterations in pulmonary vascular resistance that accompany the above changes of pneumonia, the lungs may be less able to add oxygen and remove carbon dioxide from mixed venous blood for delivery to end organs. The spread of infection or inflammatory response, either systemically or to other focal sites, further exacerbates the situation.
Viral infections are characterized by the accumulation of mononuclear cells in the submucosa and perivascular space, resulting in partial obstruction of the airway. Patients with these infections present with wheezing and crackles. Disease progresses when the alveolar type II cells lose their structural integrity and surfactant production is diminished, a hyaline membrane forms, and pulmonary edema develops.
In bacterial infections, the alveoli fill with proteinaceous fluid, which triggers a brisk influx of red blood cells (RBCs) and polymorphonuclear (PMN) cells (red hepatization) followed by the deposition of fibrin and the degradation of inflammatory cells (gray hepatization). During resolution, intra-alveolar debris is ingested and removed by the alveolar macrophages. This consolidation leads to decreased air entry and dullness to percussion; inflammation in the small airways leads to crackles.
Four stages of lobar pneumonia have been described. In the first stage, which occurs within 24 hours of infection, the lung is characterized microscopically by vascular congestion and alveolar edema. Many bacteria and few neutrophils are present. The stage of red hepatization (2-3 days), so called because of its similarity to the consistency of liver, is characterized by the presence of many erythrocytes, neutrophils, desquamated epithelial cells, and fibrin within the alveoli. In the stage of gray hepatization (2-3 days), the lung is gray-brown to yellow because of fibrinopurulent exudate, disintegration of RBCs, and hemosiderin. The final stage of resolution is characterized by resorption and restoration of the pulmonary architecture. Fibrinous inflammation may lead to resolution or to organization and pleural adhesions.
Bronchopneumonia, a patchy consolidation involving one or more lobes, usually involves the dependent lung zones, a pattern attributable to aspiration of oropharyngeal contents. The neutrophilic exudate is centered in bronchi and bronchioles, with centrifugal spread to the adjacent alveoli.
In interstitial pneumonia, patchy or diffuse inflammation involving the interstitium is characterized by infiltration of lymphocytes and macrophages. The alveoli do not contain a significant exudate, but protein-rich hyaline membranes similar to those found in adult respiratory distress syndrome (ARDS) may line the alveolar spaces. Bacterial superinfection of viral pneumonia can also produce a mixed pattern of interstitial and alveolar airspace inflammation.
Miliary pneumonia is a term applied to multiple, discrete lesions resulting from the spread of the pathogen to the lungs via the bloodstream. The varying degrees of immunocompromise in miliary tuberculosis (TB), histoplasmosis, and coccidioidomycosis may manifest as granulomas with caseous necrosis to foci of necrosis. Miliary herpesvirus, cytomegalovirus (CMV), or varicella-zoster virus infection in severely immunocompromised patients results in numerous acute necrotizing hemorrhagic lesions.
Etiology
Pneumonia can be caused by a myriad of microorganisms. Clinical suspicion of a particular offending agent is derived from clues obtained during the history and physical examination. While virtually any microorganism can lead to pneumonia, specific bacterial, viral, fungal, and mycobacterial infections are most common in previously healthy children. The age of infection, exposure history, risk factors for unusual pathogens, and immunization history all provide clues to the infecting agent.
In a prospective multicenter study of 154 hospitalized children with acute community-acquired pneumonia (CAP) in whom a comprehensive search for an etiology was sought, a pathogen was identified in 79% of children. Pyogenic bacteria accounted for 60% of cases, of which 73% were due to Streptococcus pneumoniae, while the atypical bacteria Mycoplasma pneumoniae and Chlamydophila pneumoniae were detected in 14% and 9%, respectively. Viruses were documented in 45% of children. Notably, 23% of the children had concurrent acute viral and bacterial disease. Mycoplasma pneumoniae and adenovirus coinfection can cause severe community-acquired pneumonia in children. [7]
In the study, preschool-aged children had as many episodes of atypical bacterial lower respiratory tract infections as older children. Multivariable analyses revealed that high temperature (38.4°C) within 72 hours after admission and the presence of pleural effusion were significantly associated with bacterial pneumonia. [8]
Specific etiologic agents vary based on age groups (ie, newborns, young infants, infants and toddlers, 5-year-olds, school-aged children and young adolescents, older adolescents).
Newborns
In newborns (aged 0-30 days), organisms responsible for infectious pneumonia typically mirror those responsible for early onset neonatal sepsis. This is not surprising in view of the role that maternal genitourinary and gastrointestinal tract flora play in both processes. Infections with group B Streptococcus, Listeria monocytogenes, or gram-negative rods (eg, Escherichia coli, Klebsiella pneumoniae) are common causes of bacterial pneumonia. These pathogens can be acquired in utero, via aspiration of organisms present in the birth canal, or by postnatal contact with other people or contaminated equipment.
Group B Streptococcus (GBS) was the most common bacterial isolate in most locales from the late 1960s to the late 1990s, when the impact of intrapartum chemoprophylaxis in reducing neonatal and maternal infection by this organism became evident. E coli has become the most common bacterial isolate among very low birth weight (VLBW) infants (1500 g or less) since that time. [9] Other potential bacterial organisms include the following:
-
Nontypeable Haemophilus influenzae (NTHi)
-
Other gram-negative bacilli
-
Enterococci
Some organisms acquired perinatally may not cause illness until later in infancy, including Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, CMV, and Pneumocystis carinii. C trachomatis organisms are presumably transmitted at birth during passage through an infected birth canal, although most infants are asymptomatic during the first 24 hours and develop pneumonia only after the first 2 weeks of life.
Group B streptococci infections are most often transmitted to the fetus in utero, usually as a result of colonization of the mother's vagina and cervix by the organism. Agents of chronic congenital infection, such as CMV, Treponema pallidum (the cause of pneumonia alba), Toxoplasma gondii, and others, may cause pneumonia in the first 24 hours of life. The clinical presentation usually involves other organ systems as well.
Community-acquired viral infections occur in newborns, although less commonly than in older infants. The most commonly isolated virus is respiratory syncytial virus (RSV). The transfer of maternal antibodies is important in protecting newborns and young infants from such infections, making premature infants (who may not have benefited from sufficient transfer of transplacental immunoglobulin [Ig] G]) especially vulnerable to lower-tract disease. In addition, premature infants may have chronic lung disease of prematurity, with associated hyperreactive airways, fewer functional alveoli, and baseline increased oxygen requirements.
Newborns may also be affected by the bacteria and viruses that commonly cause infections in older infants and children. Risk factors for infection include older siblings, group daycare, and lack of immunization.
Young infants
In the young infant (aged 1-3 months), continued concern about the perinatally acquired pathogens mentioned above remains. However, most bacterial pneumonia in this age group is community acquired and involves S pneumoniae, S aureus, and nontypeable H influenzae. S pneumoniae is by far the most common bacterial pathogen in this age group. Infection with any of these pyogenic bacteria may be complicated by lung abscess, parapneumonic effusions, and empyema, although S aureus is notorious for such complications. [10]
At this age, infants are incompletely immunized and remain at higher risk for H influenzae type B and pneumococcal disease, although herd immunity gained from widespread immunization of the population has been broadly protective. It is important to note that the current conjugate pneumococcal vaccine provides protection against 13 common pneumococcal types, but nonvaccine types remain problematic.
Most lower respiratory tract disease in young infants occurs during the respiratory virus season and is viral in origin, particularly in the patient with clinical bronchiolitis. The most common viral agents include RSV, parainfluenza viruses, influenza virus, adenovirus, and human metapneumovirus (hMPV).
Atypical organisms may rarely cause infection in infants. Of these, C trachomatis, U urealyticum, CMV, and P carinii are described.
Bordetella pertussis infection leads to pneumonia in as many as 20% of infected infants (as a complication of the whooping cough infection).
Among other potential atypical bacterial pathogens, U urealyticum and U parvum have been recovered from endotracheal aspirates shortly after birth in VLBW infants and have been variably associated with various adverse pulmonary outcomes, including bronchopulmonary dysplasia (BPD). [11, 12, 13, 14] Whether these organisms are causal or simply a marker of increased risk is unclear.
Infants, toddlers, and preschool-aged children
Viruses remain the most common cause of pneumonia in this age group, accounting for approximately 90% of all lower respiratory tract infections. Tsolia et al identified a viral infection among 65% of hospitalized children with community-acquired pneumonia. [15]
RSV is the most common viral pathogen, followed by parainfluenza types 1, 2, and 3 and influenza A or B. RSV infection occurs in the winter and early spring. Parainfluenza type 3 infection occurs in the spring, and types 1 and 2 occur in the fall. Influenza occurs in the winter.
Other viruses that cause pneumonia less frequently in infants and young children include adenovirus, enterovirus, rhinovirus, and coronavirus. A recent addition to this list is hMPV, which causes an illness similar to RSV and may be responsible for one third to one half of non-RSV bronchiolitis. The herpesviruses (HSV, VZV, and CMV) may rarely cause pneumonia, particularly in children with impaired immune systems.
Bacterial infections in this age group are seen on a regular basis. S pneumoniae is by far the most common bacterial cause of pneumonia. Among hospitalized children with bacterial pneumonia, S pneumoniae accounts for 21-44% of disease. [8, 16, 17] Other agents to consider include H influenzae type B (HiB) (very uncommon in immunized children [18] ), S pyogenes, and S aureus.
Children younger than 5 years, children enrolled in daycare, or those with frequent ear infections are at increased risk for invasive pneumococcal disease and infection with resistant pneumococcal strains. Evidence suggests that breastfeeding has a protective effect against invasive pneumococcal infection.
School-aged children and young adolescents
M pneumoniae is the most frequent cause of pneumonia among older children and adolescents. Mycoplasma accounts for 14-35% of pneumonia hospitalizations in this age group. [8, 15, 19] Children in homeless shelters and group homes and those with household contacts are at particular risk. Similarly, the diagnosis must be considered in immunocompromised children.
In this age group, pyogenic bacterial pneumonia remains a concern, usually caused by S pneumoniae. Other pyogenic bacterial pathogens to consider include S aureus and S pyogenes.
Chlamydophila pneumoniae also causes pneumonia. The related organism, C psittaci, is an unusual cause of pneumonia that occurs in people who work with and handle birds.
In immunosuppressed individuals, opportunistic infections with organisms such as Aspergillus species, Pneumocystis jirovecii, and CMV can occur.
Viral pneumonia remains common in this age group. Influenza pneumonia is a particular concern because ongoing infection with this virus predisposes to the development of bacterial superinfection, usually with S pneumoniae or S aureus.
Older adolescents
M pneumoniae is the most common cause of community-acquired pneumonia during the teenage and young adult years. Atypical pneumonia caused by C pneumoniae can present with identical signs and symptoms. Bacterial pneumonia caused by S pneumoniae is also seen.
Pulmonary infections caused by dimorphic fungi are also seen in this age group. Histoplasma capsulatum, which is found in nitrate-rich soil, is usually acquired as a result of inhalation of spores. Chicken coops and other bird roosts and decaying wood are often-cited sources. Cryptococcus neoformans is a common infection among pigeon breeders, but it is unusual in other immunocompetent individuals.
Blastomyces dermatitides, another dimorphic fungus, is found in certain geographic locations, most notably the Ohio and Mississippi River valleys. As with histoplasmosis, blastomycosis is acquired by inhalation of spores. Although 3 distinct forms of infection exist, the most common is acute pneumonia, which, in previously healthy individuals, most often resolves without treatment.
Viral pneumonias are common in this age group and are usually mild and self-limited, but influenza pneumonia can be severe or protracted, particularly when a bacterial infection follows.
TB pneumonia in children warrants special mention. It can occur in any age group, and it is important to remember that children with TB usually do not present with symptoms until 1-6 months after primary infection. Any child with pneumonia who has a history of TB exposure, or who has traveled to a TB-endemic area of the world needs to be fully evaluated for the possibility of tuberculosis.
Legionella pneumophila, the agent of Legionnaires disease, can also cause pneumonia, although it is uncommon in the pediatric age group.
Not all pneumonia is caused by infectious agents. Children who have severe gastroesophageal reflux (GERD) may develop chemical pneumonitis secondary to recurrent aspiration. Inhalation of certain chemicals or smoke may cause pulmonary inflammation. Additionally, aspiration pneumonia is more common among children with neurologic impairment, swallowing abnormalities, gastrointestinal motility, or a gastrostomy tube. Oral anaerobic flora, with or without aerobes, is the most common etiologic agent.
A study by Thomson et al compared hospital management and outcomes of children with neurologic impairment diagnosed with aspiration versus nonaspiration pneumonia. The study found that hospitalized children with neurologic impairment diagnosed with aspiration pneumonia had significantly longer length of stay; more ICU transfers; greater hospitalization costs; and more 30-day readmissions than did children with neurologic impairment diagnosed with nonaspiration pneumonia. [20]
Immunocompromised children
Some children who are immunocompromised, whether secondary to HIV infection/AIDS, an immune disorder, or chemotherapy for a malignancy, are at risk for pneumonias caused by opportunistic agents. Virtually any bacteria, virus, fungus, or even parasite can invade and infect the lungs if the immune system is sufficiently impaired. Carefully obtained samples for appropriate microbiologic testing are paramount in such patients so that therapy can be optimized.
Children with cystic fibrosis are especially prone to develop infections with S aureus, Pseudomonas aeruginosa, Burkholderia cepacia, and other multidrug-resistant organisms.
P jirovecii pneumonia (PCP) is common in the most severely compromised children and can lead to respiratory failure and death in those who are profoundly immunocompromised. Adenovirus infections can be severe in these children as well, leading to bronchiolitis obliterans or hyperlucent lung syndrome. In addition, CMV poses a great risk to immunocompromised patients.
Fungal pneumonia, caused by Aspergillus, Zygomycetes, or other related fungi, occurs in immunocompromised patients who undergo prolonged hospitalization, have prolonged neutropenia, and/or have received broad-spectrum antibiotics. Patients with underlying hematologic malignancies are at the highest risk.
Patients with sickle cell disease have problems with their complement system as well as functional asplenia; this predisposes them to infection with encapsulated organisms such as S pneumoniae and H influenzae type B. M pneumoniae is also a common agent of pneumonia in this group of patients.
Epidemiology
United States statistics
Pneumonia can occur at any age, although it is more common in younger children. Pneumonia accounts for 13% of all infectious illnesses in infants younger than 2 years of age. In a large community-based study conducted by Denny and Clyde, the annual incidence rate of pneumonia was 4 cases per 100 children in the preschool-aged group, 2 cases per 100 children aged 5-9 years, and 1 case per 100 children aged 9-15 years. [21]
Thompson et al reported that, after elderly persons, the second highest rates of influenza-associated hospitalizations in the United States were in children younger than 5 years of age. [22] These investigators evaluated annual influenza-associated hospitalizations by hospital discharge category, discharge type, and age group.
In a randomized double-blind trial, the heptavalent pneumococcal vaccine reduced the incidence of clinically diagnosed and radiographically diagnosed pneumonia among children younger than 5 years of age by 4% and 20%, respectively. [23] Although the overall rate of pneumonia has decreased in the United States with the use of the 7-valent vaccine, the rate of empyema and complicated pneumonia has increased. [24] Following the use of the 13-valent conjugated pneumococcal polysaccharide vaccine, the overall rates of pneumonia are anticipated to drop further. The new vaccine includes serotypes that have become associated with complicated or antibiotic-resistant disease (19A and 6A, for example).
Among school-aged children and adolescents, bronchopneumonia occurs in 0.8-2% of all pertussis cases and 16-20% of hospitalized cases. M pneumoniae accounts for 14-35% of pneumonia hospitalizations in this age group, [8, 15, 19] and mycobacterial pneumonia has recently been noted with increasing frequency in some inner-city areas, particularly among children in homeless shelters and group homes and those with household contacts.
International statistics
Pneumonia and other lower respiratory tract infections are the leading cause of death worldwide. The WHO Child Health Epidemiology Reference Group estimated the median global incidence of clinical pneumonia to be 0.28 episodes per child-year. [25] This corresponds to an annual incidence of 150.7 million new cases, of which 11-20 million (7-13%) are severe enough to require hospital admission. Ninety-five percent of all episodes of clinical pneumonia in young children worldwide occur in developing countries.
Approximately 150 million new cases of pneumonia occur annually among children younger than 5 years of age worldwide. This accounts for approximately 10-20 million hospitalizations. [21] A WHO Child Health Epidemiology Reference Group publication cited the incidence of community-acquired pneumonia among children younger than 5 years of age in developed countries as approximately 0.026 episodes per child-year. [25] In addition, a study conducted in the United Kingdom showed that 59% of deaths from pertussis are associated with pneumonia.
Prognosis
In general, the prognosis is good. Most cases of viral pneumonia resolve without treatment; common bacterial pathogens and atypical organisms respond to antimicrobial therapy. Long-term alteration of pulmonary function is rare, even in children with pneumonia that has been complicated by empyema or lung abscess.
Patients placed on a protocol-driven pneumonia clinical pathway are more likely to have favorable outcomes. The prognosis for varicella pneumonia is somewhat more guarded. Staphylococcal pneumonia, although rare, can be very serious despite treatment.
Morbidity
Although viral pneumonias are common in school-aged children and adolescents and are usually mild and self-limited, these pneumonias are occasionally severe and can rapidly progress to respiratory failure, either as a primary manifestation of viral infection or as a consequence of subsequent bacterial infection.
Morbidity and mortality from RSV and other viral infections is higher among premature infants and infants with underlying lung disease. Significant sequelae occur with adenoviral disease, including bronchiolitis obliterans and necrotizing bronchiolitis. With neonatal pneumonia, even if the infection is eradicated, many hosts develop long-lasting or permanent pulmonary changes that affect lung function, quality of life, and susceptibility to assorted subsequent infections.
Infants and postpubescent adolescents with TB pneumonia are at increased risk of disease progression. If TB is not treated during the early stages of infection, approximately 25% of children younger than 15 years of age develop extrapulmonary disease.
Bronchopneumonia occurs in 0.8-2% of all pertussis cases and 16-20% of hospitalized cases; the survival rate of these patients is much lower than in those with pneumonia that is attributed to other causes.
Immunocompromised children, those with underlying lung disease, and neonates are at high risk for severe sequelae, and they are also susceptible to various comorbidities. Cryptococcosis may occur in as many as 5-10% of patients afflicted with HIV-AIDS, and acute chest syndrome occurs in 15-43% of patients with sickle cell disease. Individuals with sickle cell disease not only have problems with their complement system, but they also have functional asplenia. These conditions predispose them to infection with encapsulated organisms such as S pneumoniae and H influenzae type B.
Mortality
The United Nations Children's Fund (UNICEF) estimates that 3 million children die worldwide from pneumonia each year; these deaths almost exclusively occur in children with underlying conditions, such as chronic lung disease of prematurity, congenital heart disease, and immunosuppression. Although most fatalities occur in developing countries, pneumonia remains a significant cause of morbidity in industrialized nations.
According to the WHO’s Global Burden of Disease 2000 Project, lower respiratory tract infections were the second leading cause of death in children younger than 5 years of age (about 2.1 million [19.6%]). [25] Most children are treated as outpatients and fully recover. However, in young infants and immunocompromised individuals, mortality is much higher. In studies of adults with pneumonia, a higher mortality rate is associated with abnormal vital signs, immunodeficiency, and certain pathogens.
Complications
Severe respiratory compromise may require intubation and transfer of the patient to a suitable intensive care unit (ICU) for more intensive monitoring and therapy. Indications for transfer include refractory hypoxia, decompensated respiratory distress (eg, lessening tachypnea due to fatigue, hypercapnia), and systemic complications such as sepsis.
Transfer may need to be initiated at a lower threshold for infants or young children, because decompensation may be rapid. Transfer of very sick infants or young children to a pediatric ICU is best accomplished with the assistance of a specialist pediatric transfer team. This may be true even if it entails a slightly longer wait, as compared with a conventional medical transport provider, or even one that provides air transport.
Severe coughing, especially in the context of necrotizing pneumonias or bullae formation, may lead to spontaneous pneumothoraces. These may or may not require treatment depending on the size of the pneumothorax. It is true, whether the pneumothorax is under tension and compromising ventilation or cardiac output.
Other complications include the following:
-
Pleural effusion
-
Pneumatocele
-
Lung abscess
-
Necrotizing pneumonia
-
Systemic infection with metastatic foci
-
Air leak syndrome, including pneumothorax, pneumomediastinum, pneumopericardium, and pulmonary interstitial emphysema
-
Airway injury
-
Obstructive airway secretions
-
Hypoperfusion
-
Chronic lung disease
-
Hypoxic-ischemic and cytokine-mediated end-organ injury
-
Sepsis
Patient Education
Counsel parents about the need to prevent exposure of infants to tobacco smoke, and, as part of anticipatory primary care, educate parents regarding possible later infectious exposures in daycare centers, schools, and similar settings. They should also be reminded of the importance of hand washing. In addition, discuss the benefit infants may receive from pneumococcal immunization and annual influenza immunization and the potential benefits and costs of RSV immune globulin. Emphasize careful longitudinal surveillance regarding long-term problems associated with growth, development, otitis, reactive airway disease, and other complications.
Most children treated with outpatient antibiotics become much improved within 48 hours following the initiation of treatment. Educate parents about and caution them to look for signs of increasing respiratory distress and to seek medical attention immediately should any of these signs appear.
For patient education resources, consult the Lung Disease and Respiratory Health Center. Also, see patient education articles on topics such as Bronchoscopy, Viral Pneumonia, and Bacterial Pneumonia.
-
(Left) Gram stain demonstrating gram-positive cocci in pairs and chains and (right) culture positive for Streptococcus pneumoniae.
-
A breakdown of test results and recommended treatment for pneumonia with effusion. Gm = Gram; neg = negative; pos = positive; VATS = video-assisted thoracic surgery
-
(A) Anteroposterior radiograph from a child with presumptive viral pneumonia. (B) Lateral radiograph of the same child with presumptive viral pneumonia.
-
Radiograph from a patient with bacterial pneumonia (same patient as in the preceding image) a few days later. This radiograph reveals progression of pneumonia into the right middle lobe and the development of a large parapneumonic pleural effusion.
-
Right lower lobe consolidation in a patient with bacterial pneumonia.
-
(A) Anteroposterior radiograph from a child with a left lower lobe infiltrate. (B) Lateral radiograph of the same child with a left lower lobe infiltrate.
-
Anteroposterior radiograph from a child with a round pneumonia.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Approach Considerations
- Complete Blood Cell Count
- Sputum Gram Stain and Culture
- Blood Culture
- Serology
- Inflammatory Markers
- Polymerase Chain Reaction
- Skin Testing
- Gastric Aspirates
- Cold Agglutinin Testing
- Urine Latex Agglutination Testing
- Direct Antigen Detection
- Imaging Studies
- Bronchoscopy
- Bronchoscopic Alveolar Lavage
- Protected Brush Tracheal Aspirate Sampling
- Lung Aspiration
- Lung Puncture
- Thoracentesis
- Histology
- Show All
- Treatment
- Medication
- Questions & Answers
- Media Gallery
- Tables
- References