Background
Achondroplasia, a nonlethal form of chondrodysplasia, is the most common type of short-limb dwarfism. This skeletal dysplasia is inherited as a Mendelian autosomal dominant trait with complete penetrance. Approximately 80% of cases are due to a new (de novo) dominant mutation, with the mutation rate estimated to be 1.4 x 10-5 per gamete per generation. [1] Salient phenotypic features include disproportionate short stature, megalencephaly, a prominent forehead (frontal bossing), midface hypoplasia, a normal trunk length, rhizomelic (proximal) shortening of the arms and legs, prominent lumbar lordosis, genu varum (bowed legs), and a trident-hand configuration. [2] Graphs illustrating linear growth (height), growth velocity, upper and lower segment length, and head circumference (occipitofrontal circumference [OFC]), for both females and males diagnosed with achondroplasia, are shown below.
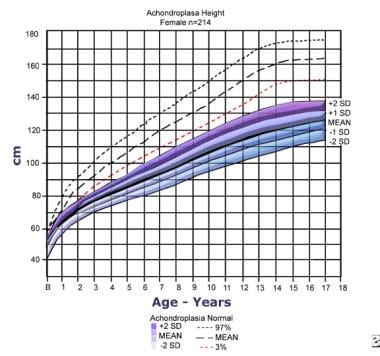 Height for females with achondroplasia (mean/standard deviation [SD]) compared to normal standard curves. The graph is based on information from 214 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
Height for females with achondroplasia (mean/standard deviation [SD]) compared to normal standard curves. The graph is based on information from 214 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
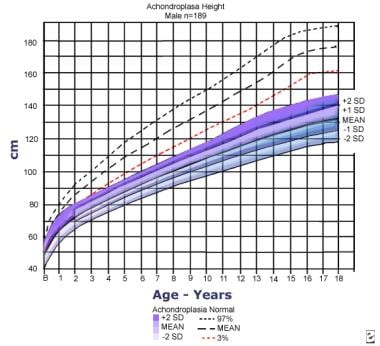 Height for males with achondroplasia (mean/2 standard deviations [SDs]) compared to normal standard curves. The graph is based on information from 189 males. Adapted from Horton WA, Rotter JI, Rimoin DL, et al: Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
Height for males with achondroplasia (mean/2 standard deviations [SDs]) compared to normal standard curves. The graph is based on information from 189 males. Adapted from Horton WA, Rotter JI, Rimoin DL, et al: Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
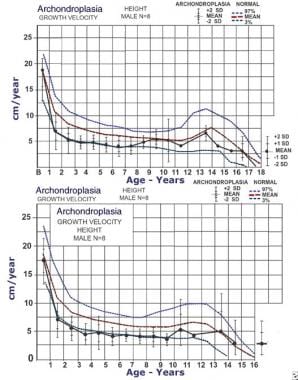 Mean growth velocities (solid line) for males (top) and females (bottom) with achondroplasia compared to normal growth velocity curves. Dashed lines indicate third percentile, mean, and 97th percentile. Data are from 26 males and 35 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
Mean growth velocities (solid line) for males (top) and females (bottom) with achondroplasia compared to normal growth velocity curves. Dashed lines indicate third percentile, mean, and 97th percentile. Data are from 26 males and 35 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
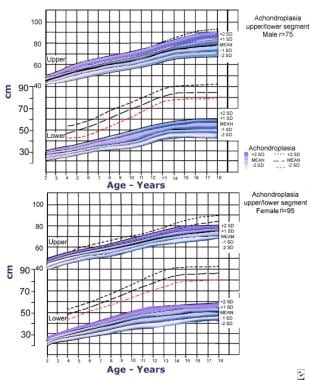 Upper and lower segment lengths for males (top) and (bottom) with achondroplasia (mean/standard deviation [SD]). Data are from 75 males and 95 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
Upper and lower segment lengths for males (top) and (bottom) with achondroplasia (mean/standard deviation [SD]). Data are from 75 males and 95 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
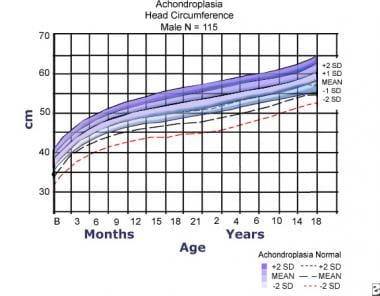
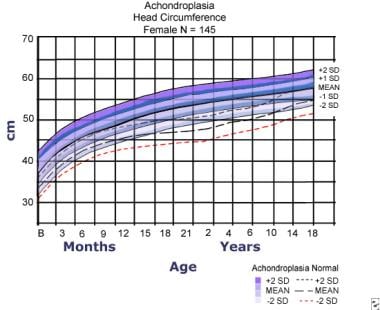 Head circumference for females with achondroplasia compared to normal curves (dashed lines). Data are from 145 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
Head circumference for females with achondroplasia compared to normal curves (dashed lines). Data are from 145 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
Pathophysiology
Achondroplasia is caused by mutations in the fibroblast growth factor receptor-3 (FGFR3) gene. [2, 3, 4, 5, 6, 7, 8, 9] Mutations within FGFR3 are the only genetic changes known to cause achondroplasia. [10] FGFR3 has been mapped to the short arm of chromosome 4, p16.3 (4p16.3). [11, 12] All causal mutations occur at the exact same location within the gene; hence, molecular testing by targeted mutational analysis is easily done and interpreted. The two mutations, G1138A and G1138C, cause increased function of the FGFR3 gene. These mutations cause decreased endochondral ossification, decreased cellular hypertrophy, decreased cartilage matrix production, and inhibited proliferation of chondrocytes in growth plate cartilage.
G1138A and G1138C mutations of FGFR3 account for 99% of the mutational changes in patients with achondroplasia. A specific point mutation results; hence, an amino acid substitution occurs. [13] About 98% of diagnosed patients have the G1138A mutation, resulting in a G-to-A DNA nucleotide point change. One percent of cases have a G-to-C DNA point change at nucleotide 1138, causing the G1138C mutation. A rare missense mutation (Lys650Met) in the tyrosine kinase region of FGFR3 causes a disorder termed severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN). [14] See Differentials.
A study by Di Rocco et al using murine and human subjects indicated that FGFR3 mutations in achondroplasia also affect membranous ossification. The investigators analyzed the calvaria and skull base in mice with an achondroplasia-like mutation, as well as in humans with achondroplasia or FGFR3-related craniosynostoses. Their evaluation revealed abnormal cartilage and premature fusion of the synchondroses, which changed the size and shape of the foramen magnum. [15]
Epidemiology
Frequency
United States
Frequency has not been documented for births in the United States.
International
Frequency is believed to be 1 case per 15,000-40,000 births worldwide, with an average worldwide frequency of 1 in 25,000 live births. However, there is a wide range, with the frequency of achondroplasia estimated to be 1 in 6400 live births in Denmark, and 1 in 10,000 live births in Latin America.
In 1986, Orioli et al reported on the frequency of all skeletal dysplasias in a study population of 349,470 live births and stillbirths. [16] Based on this study, the prevalence rate for achondroplasia was estimated to be 0.5-1.5 cases per 10,000 births (1 in 20,000 to 1 in 6,666 births) and the mutation rate to be 1.72-5.57 x 10-5 per gamete per generation.
Mortality/Morbidity
Central nervous system
Sudden death within the first year of life is attributed to abnormalities at the craniocervical junction leading to spinal cord compression. Central apnea occurs as a result of arterial compression at the cervical level of the foramen magnum. A small foramen magnum present in these patients may also cause a high cervical myelopathy.
The risk of sudden death for infants with achondroplasia is 2-5%. This risk can be minimized with appropriate assessment of the craniocervical junction, which includes a thorough neurological history and examination, neuroimaging (either computed tomography [CT] scanning or magnetic resonance imaging [MRI]), and polysomnography (sleep study). The presence of neurological abnormalities necessitates referral to a medical center with neurosurgical consultation services.
Cervical instability is present in a large number of patients. Great care must be taken with manipulation of the neck, as would occur for preparation of intubation in general anesthesia. Uncontrolled neck movements can cause significant neurological compromise with spinal cord compression.
Parents and caregivers should use an infant carrier with a firm back that affords good neck support and use a rear-facing car seat for travel as long as possible. Use of mechanical swings and carrying slings should be avoided to prevent uncontrolled head movement.
Thoracolumbar kyphosis occurs in most infants with achondroplasia. Severe kyphosis is related to unsupported sitting of the infant before adequate trunk muscle strength has developed. [17] Angular deformities of the extremities, premature degenerative joint disease, and spinal disorders are seen in these patients.
School-aged children with achondroplasia have shown head CT-scan findings of neuroanatomic abnormalities consistent with arrested hydrocephalus. In addition, enlarged ventricles and hypoplasia of the corpus callosum have been noted. These CT-scan findings are similar to those observed in children with compensated, unshunted hydrocephalus. The hydrocephalus may be due to increased intracranial venous pressure secondary to stenosis of the sigmoid sinus at the level of the narrowed jugular foramina.
Pulmonology and apnea
Respiratory disorders are seen frequently, including apnea and abnormalities of pulmonary gas exchange. Up to 75% of children with achondroplasia demonstrate a pathologic apnea index (> 30 episodes). Brainstem compression may contribute to central apnea. Obstructive sleep apnea may be due to midface hypoplasia and high body mass index. [18, 19] See Nutrition and body mass index (BMI) below.
Severe upper airway obstruction occurs in less than 5% in children with achondroplasia. For these children, tonsillectomy and adenoidectomy do not markedly resolve this obstruction. Adenotonsillar hypertrophy, hypotonicity, and a narrow trunk with a small thoracic cage all contribute to confining the airway and causing upper airway obstruction.
Children with achondroplasia who have respiratory dysfunction and obstructive sleep apnea (OSA) detected by polysomnography have associated cognitive deficits, as reported in children with OSA within the general population. Restrictive pulmonary disease, with or without restrictive airway disease, occurs in less than 5% of young children (< 3 y). This risk increases for patients who live at higher elevations.
Development
Children with achondroplasia have cognitive scores within normal; however, they may have mild deficits in visual-spatial tasks. This deficit has also been identified in children with arrested hydrocephalus.
Motor milestones typically are delayed for the first year of life due to a large cranium and poor overall muscle tone (hypotonia). Language development is normal, if no conductive hearing loss is present. [20]
Nutrition and body mass index (BMI)
Obesity, when present, aggravates the morbidity related to lumbar stenosis, nonspecific joint problems, and cardiovascular and apnea risks. Based on the weight/height (W/H) curves developed by Hunter et al for boys and girls with achondroplasia, the mean W/H curve in children with achondroplasia matches the control curve until the children reach 75 cm in height. Beyond 75 cm, the weight in children with achondroplasia increases disproportionately to height.
The Quetelet index or body mass index (BMI=W/H2) can be used to estimate weight excess in children aged 3-6 years. After that, the Rohrer index (RI=W/H3) should be used for children and adolescents aged 6-18 years; this index can be further extrapolated to adults.
Race
No documented race predilection is noted.
Sex
Males and females are equally affected, as the FGFR3 gene is located on chromosome 4 (an autosome) and not on a sex chromosome.
-
Height for females with achondroplasia (mean/standard deviation [SD]) compared to normal standard curves. The graph is based on information from 214 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
-
Height for males with achondroplasia (mean/2 standard deviations [SDs]) compared to normal standard curves. The graph is based on information from 189 males. Adapted from Horton WA, Rotter JI, Rimoin DL, et al: Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
-
Mean growth velocities (solid line) for males (top) and females (bottom) with achondroplasia compared to normal growth velocity curves. Dashed lines indicate third percentile, mean, and 97th percentile. Data are from 26 males and 35 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
-
Upper and lower segment lengths for males (top) and (bottom) with achondroplasia (mean/standard deviation [SD]). Data are from 75 males and 95 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
-
Head circumference for females with achondroplasia compared to normal curves (dashed lines). Data are from 145 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.
-
Head circumference for males with achondroplasia compared to normal curves (dashed lines). Data are from 114 females. Adapted from Horton WA, Rotter JI, Rimoin DL, et al. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep; 93(3): 435-8.